To describe the distribution of electrons around the atomic nucleus, physicists use a simple historical notation: the electron shells K, L, M, N, O, P, and Q. This notation was introduced in the early 20th century by physicist Charles Barkla (1877-1944) during the study of X-rays. This notation allows for a quick visualization of how electrons are distributed by increasing energy levels, from the shell closest to the nucleus (K) to the outer shells.
Each letter corresponds to a principal quantum number n:
K Shell: n = 1 (first shell, closest to the nucleus)
L Shell: n = 2 (second shell)
M Shell: n = 3 (third shell)
N Shell: n = 4 (fourth shell)
O Shell: n = 5 (fifth shell)
P Shell: n = 6 (sixth shell)
Q Shell: n = 7 (seventh shell)
Each shell can contain a maximum number of electrons defined by the formula 2n²:
K Shell (n=1): maximum 2 electrons (2 × 1² = 2)
L Shell (n=2): maximum 8 electrons (2 × 2² = 8)
M Shell (n=3): maximum 18 electrons (2 × 3² = 18)
N Shell (n=4): maximum 32 electrons (2 × 4² = 32) → Uranium reaches this: K(2) L(8) M(18) N(32)
O Shell (n=5): maximum 50 electrons (2 × 5² = 50) → Never reached (uranium has only 21 electrons in O)
P Shell (n=6): maximum 72 electrons (2 × 6² = 72) → Never reached
Q Shell (n=7): maximum 98 electrons (2 × 7² = 98) → Never reached
N.B.:
In practice, no known element completely fills the shells beyond N. The heaviest natural element, uranium (Z=92), has the configuration K(2) L(8) M(18) N(32) O(21) P(9) Q(2). The heaviest confirmed synthetic element, oganesson (Z=118), has the configuration K(2) L(8) M(18) N(32) O(32) P(18) Q(8).
Each shell is divided into subshells designated by the letters s, p, d, f:
s Subshell: can contain up to 2 electrons (1 orbital)
p Subshell: can contain up to 6 electrons (3 orbitals)
d Subshell: can contain up to 10 electrons (5 orbitals)
f Subshell: can contain up to 14 electrons (7 orbitals)
K Shell (n=1): contains only 1s (2 electrons max)
L Shell (n=2): contains 2s and 2p (2 + 6 = 8 electrons max)
M Shell (n=3): contains 3s, 3p, and 3d (2 + 6 + 10 = 18 electrons max)
N Shell (n=4): contains 4s, 4p, 4d, and 4f (2 + 6 + 10 + 14 = 32 electrons max)
O Shell (n=5): contains 5s, 5p, 5d, and 5f (2 + 6 + 10 + 14 = 32 electrons max theoretical, although the theoretical 5g subshell does not exist in known elements)
P Shell (n=6): contains 6s, 6p, 6d, and 6f (2 + 6 + 10 + 14 = 32 electrons max for known subshells)
Q Shell (n=7): contains 7s, 7p, and potentially 7d (only 7s and 7p electrons are observed in known elements)
This notation indicates the total number of electrons present in each shell, without detailing the subshells. It is particularly useful for quickly visualizing the overall electronic distribution of an atom.
Helium (2 electrons): 1s² → K(2)
The K shell is complete and saturated.
Neon (10 electrons): 1s² 2s² 2p⁶ → K(2) L(8)
The K and L shells are complete and saturated.
Sodium (11 electrons): 1s² 2s² 2p⁶ 3s¹ → K(2) L(8) M(1)
The K and L shells are complete, the M shell contains only 1 electron out of 18 possible.
Argon (18 electrons): 1s² 2s² 2p⁶ 3s² 3p⁶ → K(2) L(8) M(8)
The K and L shells are complete. The M shell contains 8 electrons but is not complete (the 3s and 3p subshells are saturated, but 3d remains empty).
Calcium (20 electrons): 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² → K(2) L(8) M(8) N(2)
Note that the 4s subshell fills before the 3d, which is why the M shell remains at 8 electrons.
Titanium (22 electrons): 1s² 2s² 2p⁶ 3s² 3p⁶ 3d² 4s² → K(2) L(8) M(10) N(2)
The M shell begins to fill with 3d electrons.
The filling order does not strictly follow the order of K, L, M, N shells… due to the energy levels of the subshells. The general order is:
1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d → 5p → 6s → 4f → 5d → 6p → 7s → 5f → 6d…
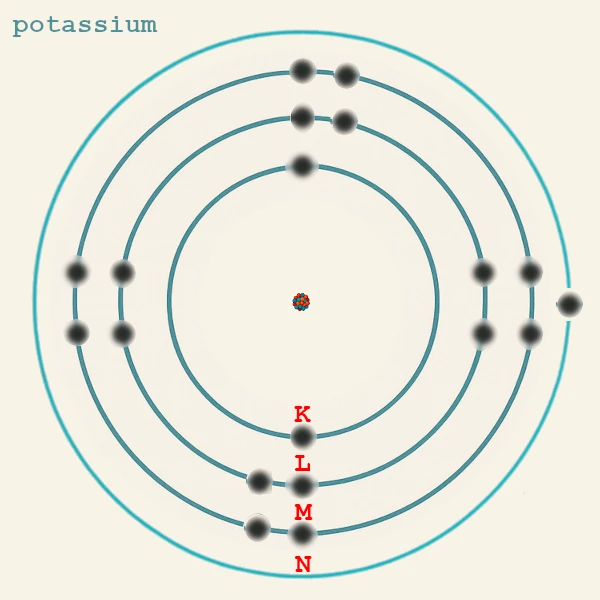
This principle explains why, for example, potassium (19 electrons) has the configuration K(2) L(8) M(8) N(1): the 19th electron goes into 4s rather than 3d because the 4s subshell is of lower energy than 3d.
The K, L, M, N, O, P, Q notation allows:
• Quick visualization of the overall electronic structure of an atom
• Easy identification of the valence shell (outer shell)
• Understanding of chemical properties related to valence electrons
• Explanation of the classification of elements in the periodic table
• Prediction of oxidation states and chemical reactivity of elements
The chemical reactivity of elements originates from their electronic structure, particularly the configuration of their valence shell. The outer shell, or valence shell, determines an element's ability to form chemical bonds: atoms tend to gain, lose, or share electrons to achieve a stable configuration, usually that of the nearest noble gas.
This tendency explains the observed chemical affinities: alkali metals, with a single valence electron, readily give it up to form cations; halogens, which lack one electron to complete their outer shell, are electron-hungry and form anions; while noble gases, with a complete valence shell, remain chemically inert. Between these extremes, transition elements and metalloids exhibit intermediate behaviors, forming various types of bonds depending on conditions.
Understanding these structure-property relationships is the foundation of modern chemistry and allows the prediction of element behavior in chemical reactions.
| Element | Symbol (Z) | Valence shell | Valence electrons | Valence configuration | Affinity with other elements (examples) |
|---|---|---|---|---|---|
| Hydrogen | H (Z = 1) | n = 1 | 1 | 1s¹ | Covalent bonds with O (H₂O), C (CH₄), N (NH₃). |
| Helium | He (Z = 2) | n = 1 | 2 | 1s² | Almost no chemical affinity; inert noble gas. |
| Lithium | Li (Z = 3) | n = 2 | 1 | 2s¹ | Ionic bonds with F (LiF), O (Li₂O). |
| Beryllium | Be (Z = 4) | n = 2 | 2 | 2s² | Covalent bonds with O (BeO), C (Be₂C). |
| Boron | B (Z = 5) | n = 2 | 3 | 2s² 2p¹ | Deficient bonds with F (BF₃), H (boranes). |
| Carbon | C (Z = 6) | n = 2 | 4 | 2s² 2p² | Covalent bonds with H, O (CO₂), N. |
| Nitrogen | N (Z = 7) | n = 2 | 5 | 2s² 2p³ | Multiple bonds with H (NH₃), C (CN⁻). |
| Oxygen | O (Z = 8) | n = 2 | 6 | 2s² 2p⁴ | Major oxidant; bonds with H, C, metals. |
| Fluorine | F (Z = 9) | n = 2 | 7 | 2s² 2p⁵ | Very high electronegativity; ionic bonds. |
| Neon | Ne (Z = 10) | n = 2 | 8 | 2s² 2p⁶ | Inert noble gas. |
| Sodium | Na (Z = 11) | n = 3 | 1 | 3s¹ | Ionic bonds with Cl (NaCl), O. |
| Magnesium | Mg (Z = 12) | n = 3 | 2 | 3s² | Ionic bonds with O (MgO). |
| Aluminum | Al (Z = 13) | n = 3 | 3 | 3s² 3p¹ | Metallic and covalent bonds (Al₂O₃). |
| Silicon | Si (Z = 14) | n = 3 | 4 | 3s² 3p² | Covalent networks (SiO₂). |
| Phosphorus | P (Z = 15) | n = 3 | 5 | 3s² 3p³ | Bonds with O (PO₄³⁻), H. |
| Sulfur | S (Z = 16) | n = 3 | 6 | 3s² 3p⁴ | Bonds with H (H₂S), O (SO₂). |
| Chlorine | Cl (Z = 17) | n = 3 | 7 | 3s² 3p⁵ | Ionic bonds with Na, covalent with H. |
| Argon | Ar (Z = 18) | n = 3 | 8 | 3s² 3p⁶ | Inert noble gas. |
| Potassium | K (Z = 19) | n = 4 | 1 | 4s¹ | Ionic bonds with Cl (KCl), O. |
| Calcium | Ca (Z = 20) | n = 4 | 2 | 4s² | Ionic bonds with O (CaO), carbonates. |
| Scandium | Sc (Z = 21) | n = 4 | 3 | 4s² 3d¹ | Metallic bonds; oxides Sc₂O₃. |
| Titanium | Ti (Z = 22) | n = 4 | 4 | 4s² 3d² | Metallic bonds; oxides TiO₂. |
| Vanadium | V (Z = 23) | n = 4 | 5 | 4s² 3d³ | Multiple oxidation states; V₂O₅. |
| Chromium | Cr (Z = 24) | n = 4 | 6 | 4s¹ 3d⁵ | Metallic bonds; oxides Cr₂O₃. |
| Manganese | Mn (Z = 25) | n = 4 | 7 | 4s² 3d⁵ | Varied oxidation states; MnO₂. |
| Iron | Fe (Z = 26) | n = 4 | 2–3 | 4s² 3d⁶ | Metallic bonds; oxides Fe₂O₃. |
| Cobalt | Co (Z = 27) | n = 4 | 2–3 | 4s² 3d⁷ | Metallic bonds; complexes CoO. |
| Nickel | Ni (Z = 28) | n = 4 | 2 | 4s² 3d⁸ | Metallic bonds; catalyst NiO. |
| Copper | Cu (Z = 29) | n = 4 | 1–2 | 4s¹ 3d¹⁰ | Metallic bonds; oxides CuO. |
| Zinc | Zn (Z = 30) | n = 4 | 2 | 4s² 3d¹⁰ | Ionic bonds with O, S. |
| Gallium | Ga (Z = 31) | n = 4 | 3 | 4s² 4p¹ | Metallic bonds; semiconductors GaAs. |
| Germanium | Ge (Z = 32) | n = 4 | 4 | 4s² 4p² | Semiconductor; covalent bonds GeO₂. |
| Arsenic | As (Z = 33) | n = 4 | 5 | 4s² 4p³ | Covalent bonds with S, O. |
| Selenium | Se (Z = 34) | n = 4 | 6 | 4s² 4p⁴ | Analogous to sulfur; H₂Se. |
| Bromine | Br (Z = 35) | n = 4 | 7 | 4s² 4p⁵ | Halogen; bonds with H, metals. |
| Krypton | Kr (Z = 36) | n = 4 | 8 | 4s² 4p⁶ | Almost inert noble gas. |
| Rubidium | Rb (Z = 37) | n = 5 | 1 | 5s¹ | Alkali metal; ionic bonds RbCl. |
| Strontium | Sr (Z = 38) | n = 5 | 2 | 5s² | Ionic bonds with O (SrO). |
| Yttrium | Y (Z = 39) | n = 5 | 3 | 5s² 4d¹ | Transition metal; Y₂O₃. |
| Zirconium | Zr (Z = 40) | n = 5 | 4 | 5s² 4d² | Metallic bonds; ZrO₂. |
| Niobium | Nb (Z = 41) | n = 5 | 5 | 5s¹ 4d⁴ | Superconductor; Nb₂O₅. |
| Molybdenum | Mo (Z = 42) | n = 5 | 6 | 5s¹ 4d⁵ | Metallic bonds; MoS₂. |
| Technetium | Tc (Z = 43) | n = 5 | 7 | 5s² 4d⁵ | Radioactive; complexes with O. |
| Ruthenium | Ru (Z = 44) | n = 5 | 8 | 5s¹ 4d⁷ | Catalyst; RuO₂. |
| Rhodium | Rh (Z = 45) | n = 5 | 9 | 5s¹ 4d⁸ | Catalyst; Rh₂O₃. |
| Palladium | Pd (Z = 46) | n = 5 | 10 | 4d¹⁰ | Catalyst; PdO. |
| Silver | Ag (Z = 47) | n = 5 | 1 | 5s¹ 4d¹⁰ | Metallic bonds; Ag₂O. |
| Cadmium | Cd (Z = 48) | n = 5 | 2 | 5s² 4d¹⁰ | Ionic bonds; CdS. |
| Indium | In (Z = 49) | n = 5 | 3 | 5s² 5p¹ | Semiconductor; In₂O₃. |
| Tin | Sn (Z = 50) | n = 5 | 4 | 5s² 5p² | Metallic bonds; SnO₂. |
| Antimony | Sb (Z = 51) | n = 5 | 5 | 5s² 5p³ | Semimetal; Sb₂O₃. |
| Tellurium | Te (Z = 52) | n = 5 | 6 | 5s² 5p⁴ | Semimetal; H₂Te. |
| Iodine | I (Z = 53) | n = 5 | 7 | 5s² 5p⁵ | Halogen; bonds with H, metals. |
| Xenon | Xe (Z = 54) | n = 5 | 8 | 5s² 5p⁶ | Noble gas; compounds with F (XeF₄). |
| Cesium | Cs (Z = 55) | n = 6 | 1 | 6s¹ | Highly reactive alkali metal; CsCl. |
| Barium | Ba (Z = 56) | n = 6 | 2 | 6s² | Ionic bonds; BaO. |
| Lanthanum | La (Z = 57) | n = 6 | 3 | 6s² 5d¹ | Lanthanide; La₂O₃. |
| Cerium | Ce (Z = 58) | n = 6 | 3–4 | 6s² 4f¹ 5d¹ | Lanthanide; CeO₂. |
| Praseodymium | Pr (Z = 59) | n = 6 | 3 | 6s² 4f³ | Lanthanide; Pr₂O₃. |
| Neodymium | Nd (Z = 60) | n = 6 | 3 | 6s² 4f⁴ | Powerful magnets; Nd₂O₃. |
| Promethium | Pm (Z = 61) | n = 6 | 3 | 6s² 4f⁵ | Radioactive; Pm₂O₃. |
| Samarium | Sm (Z = 62) | n = 6 | 2–3 | 6s² 4f⁶ | Magnets; Sm₂O₃. |
| Europium | Eu (Z = 63) | n = 6 | 2–3 | 6s² 4f⁷ | Phosphor; Eu₂O₃. |
| Gadolinium | Gd (Z = 64) | n = 6 | 3 | 6s² 4f⁷ 5d¹ | Magnetic; Gd₂O₃. |
| Terbium | Tb (Z = 65) | n = 6 | 3 | 6s² 4f⁹ | Phosphor; Tb₂O₃. |
| Dysprosium | Dy (Z = 66) | n = 6 | 3 | 6s² 4f¹⁰ | Magnets; Dy₂O₃. |
| Holmium | Ho (Z = 67) | n = 6 | 3 | 6s² 4f¹¹ | Magnetic; Ho₂O₃. |
| Erbium | Er (Z = 68) | n = 6 | 3 | 6s² 4f¹² | Optical; Er₂O₃. |
| Thulium | Tm (Z = 69) | n = 6 | 3 | 6s² 4f¹³ | Lasers; Tm₂O₃. |
| Ytterbium | Yb (Z = 70) | n = 6 | 2–3 | 6s² 4f¹⁴ | Yb₂O₃. |
| Lutetium | Lu (Z = 71) | n = 6 | 3 | 6s² 4f¹⁴ 5d¹ | Lu₂O₃. |
| Hafnium | Hf (Z = 72) | n = 6 | 4 | 6s² 4f¹⁴ 5d² | Metallic bonds; HfO₂. |
| Tantalum | Ta (Z = 73) | n = 6 | 5 | 6s² 4f¹⁴ 5d³ | Resistant; Ta₂O₅. |
| Tungsten | W (Z = 74) | n = 6 | 6 | 6s² 4f¹⁴ 5d⁴ | High melting point; WO₃. |
| Rhenium | Re (Z = 75) | n = 6 | 7 | 6s² 4f¹⁴ 5d⁵ | Catalyst; Re₂O₇. |
| Osmium | Os (Z = 76) | n = 6 | 8 | 6s² 4f¹⁴ 5d⁶ | Very dense; OsO₄. |
| Iridium | Ir (Z = 77) | n = 6 | 9 | 6s² 4f¹⁴ 5d⁷ | Resistant; IrO₂. |
| Platinum | Pt (Z = 78) | n = 6 | 10 | 6s¹ 4f¹⁴ 5d⁹ | Catalyst; PtO₂. |
| Gold | Au (Z = 79) | n = 6 | 1 | 6s¹ 4f¹⁴ 5d¹⁰ | Noble; Au₂O₃. |
| Mercury | Hg (Z = 80) | n = 6 | 2 | 6s² 4f¹⁴ 5d¹⁰ | Liquid; HgO. |
| Thallium | Tl (Z = 81) | n = 6 | 3 | 6s² 6p¹ | Toxic; Tl₂O. |
| Lead | Pb (Z = 82) | n = 6 | 4 | 6s² 6p² | Metallic bonds; PbO. |
| Bismuth | Bi (Z = 83) | n = 6 | 5 | 6s² 6p³ | Semimetal; Bi₂O₃. |
| Polonium | Po (Z = 84) | n = 6 | 6 | 6s² 6p⁴ | Radioactive; PoO₂. |
| Astatine | At (Z = 85) | n = 6 | 7 | 6s² 6p⁵ | Radioactive; rare halogen. |
| Radon | Rn (Z = 86) | n = 6 | 8 | 6s² 6p⁶ | Radioactive noble gas. |
| Francium | Fr (Z = 87) | n = 7 | 1 | 7s¹ | Radioactive; alkali metal. |
| Radium | Ra (Z = 88) | n = 7 | 2 | 7s² | Radioactive; RaO. |
| Actinium | Ac (Z = 89) | n = 7 | 3 | 7s² 6d¹ | Actinide; Ac₂O₃. |
| Thorium | Th (Z = 90) | n = 7 | 4 | 7s² 6d² | Actinide; ThO₂. |
| Protactinium | Pa (Z = 91) | n = 7 | 5 | 7s² 5f² 6d¹ | Radioactive; Pa₂O₅. |
| Uranium | U (Z = 92) | n = 7 | 3–6 | 7s² 6d¹ 5f³ | Complex bonds with O (UO₂²⁺), F (UF₆). |