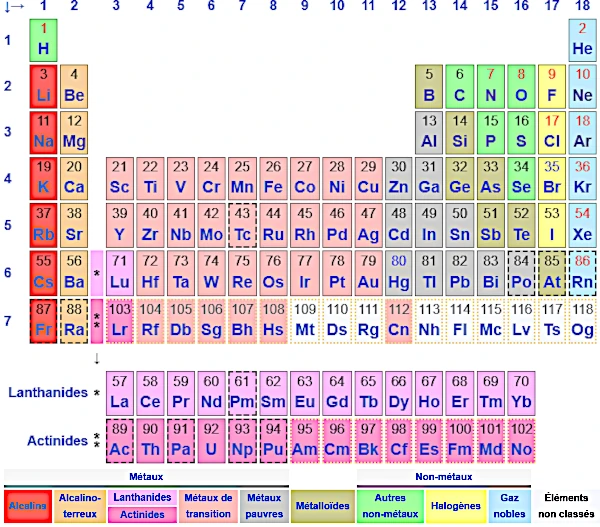
The Periodic Table of Chemical Elements, one of the most important tools in chemistry, was developed by Dmitri Mendeleev (1834-1907) in 1869. Its original version classified the 63 known elements at the time by increasing atomic mass and highlighted the periodicity of their chemical properties. Mendeleev's great innovation was to leave blank spaces for elements unknown at the time, whose properties he predicted with remarkable accuracy.
| Element | Symbol | Atomic No. | Discovery | Modern Uses |
|---|---|---|---|---|
| Helium | He | 2 | 1868 (solar spectroscopy) | Balloons, MRI, welding |
| Gallium | Ga | 31 | 1875 | Semiconductors (electronic chips) |
| Germanium | Ge | 32 | 1886 | Optical fibers, infrared lenses |
| Radium | Ra | 88 | 1898 (Pierre and Marie Curie) | Formerly in medicine (radiotherapy) |
| Neon | Ne | 10 | 1898 | Lighting (neon signs) |
| Plutonium | Pu | 94 | 1940 | Nuclear energy (civil and military) |
| Technetium | Tc | 43 | 1937 (1st artificial element) | Nuclear medicine (scanners) |
| Francium | Fr | 87 | 1939 | Fundamental research (very rare) |
| Uranium | U | 92 | 1789 (but properties unknown) | Nuclear power plants, armaments |
| Platinum | Pt | 78 | Known but not studied | Automobile catalysts, jewelry |
Mendeleev had predicted with remarkable accuracy the properties of gallium (which he called "eka-aluminum"), germanium ("eka-silicon"), and scandium ("eka-boron"). These subsequent discoveries validated his periodic table.
The modern table organizes the 118 known elements (to date) according to their atomic number (number of protons) and their recurring chemical properties. It consists of:
The elements are classified into three main categories:
Certain groups have particularly similar chemical properties:
Elements beyond uranium (atomic number > 92) are generally synthetic, produced in laboratories. They are often very unstable and rapidly decay through radioactivity.