Atoms are stable when the number of neutrons in the nucleus is roughly equal to the number of protons. When this difference is too great, the atom becomes unstable.
Heavy nuclei of neutron-rich isotopes with an atomic number greater than that of iron (N = 26) are formed at very high temperatures (>109 K) during the explosion of massive stars (supernovae).
However, all nuclei seek a more economical energy stability. Thus, to return to a stable state, they must transmute by expelling energy in the form of mass or radiation. This leads to the spontaneous decay of the nucleus, known as radioactivity.
Radioactivity is therefore a natural random phenomenon occurring in the nucleus, deep within the atom, and results in a new, more stable nucleus.
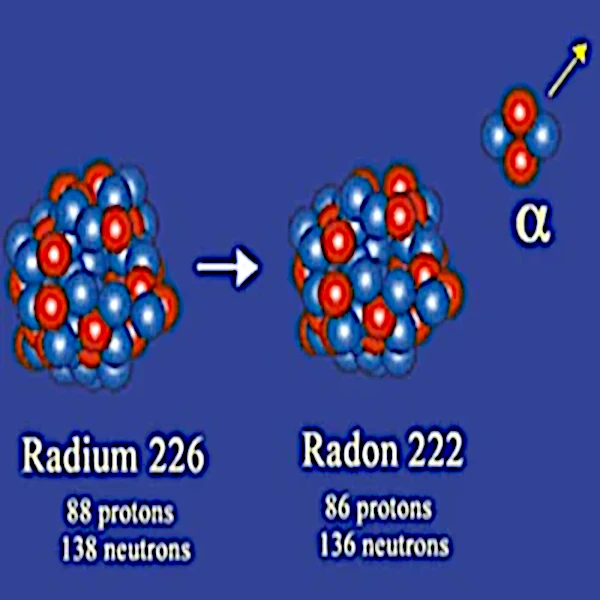
In 1908, Ernest Rutherford (1871-1937) identified alpha particles as helium-4 nuclei composed of 2 protons and 2 neutrons. Thus, the stable nucleus resulting from radioactive decay has two fewer neutrons and two fewer protons than the unstable nucleus from which it originated.
Alpha particle emission mainly concerns very large nuclei such as radium-226 (88 protons and 138 neutrons), thorium-232 (90 protons and 142 neutrons), uranium-238 (92 protons and 136 neutrons), etc.
Such nuclei are unstable because the Coulomb repulsion between protons, which increases as the square of the number of protons, is stronger than the nuclear attraction between nucleons. At this point, the nucleus expels a bundle of four nucleons.
For matter, this is the quickest and most economical way to regain stability.
Radium has no stable isotopes. The predominant radium isotope, discovered by Pierre and Marie Curie on December 21, 1898 by extracting from pitchblende (uranium ore), is radium-226, which has a radioactive period or half-life of 1,600 years (the time required for half of the initially present nuclei to transform into other nuclei).
Radium has remarkable natural properties (radioluminescence, spontaneous heat production, radioactive source, etc.). Additionally, it makes the atmosphere electrically conductive and discharges capacitors remotely, leading to the development of the Geiger-Müller counter. The Geiger counter, developed in 1928, is used to measure a wide range of ionizing radiation (alpha, beta, gamma radiation, and X-rays).
Radium was used until 1950 for its radioluminescence properties, particularly in paints intended for watchmaking. From the 1920s, occupational diseases were identified among workers who refined their radium-enriched paintbrushes by placing them in their mouths. These diseases led to an initial epidemiological investigation and the gradual closure of factories.
Due to their large mass and charge, alpha particles are not very penetrating. They can generally be stopped by the skin. However, if ingested, they become dangerous. Sufficiently high doses can cause cancer.
Note: Ionization is the process of removing or adding electromagnetic charges to an atom.