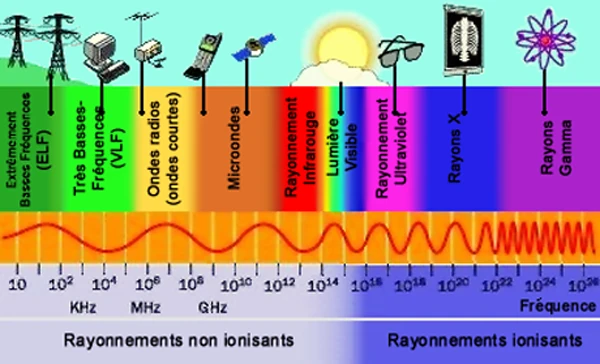
The electromagnetic spectrum includes all forms of electromagnetic radiation, classified according to their wavelength or frequency. It ranges from radio waves > 1 meter, used for communications, to gamma rays < 10-12 meters, emitted by the most energetic phenomena in the Universe.
Each band of the spectrum has unique properties exploited in various fields: microwaves for heating and radar, infrared for thermal imaging, visible light for optics, X-rays for medicine, and gamma rays for astrophysics. HVDC systems also use specific waves for high-voltage energy transport.
| Band | Wavelength | Frequency | Applications | Comment (harmful effects on humans) |
|---|---|---|---|---|
| Radio waves | > 1 m | < 300 MHz | Radio, television, mobile telephony | Slightly harmful; non-ionizing, minimal risk except for prolonged exposure to high power |
| Microwaves | 1 mm – 1 m | 300 MHz – 300 GHz | Radar, microwave oven, HVDC | Slightly harmful at low power; can cause burns or tissue heating at high intensity |
| Infrared | 700 nm – 1 mm | 300 GHz – 430 THz | Thermal imaging, remote controls | Non-ionizing; prolonged exposure can cause burns or eye damage |
| Visible | 400 – 700 nm | 430 – 750 THz | Human vision, optics | Slightly harmful; intense exposure to concentrated light can damage the retina |
| Ultraviolet | 10 – 400 nm | 750 THz – 30 PHz | Sterilization, astronomy | Partially ionizing; can cause sunburn, skin aging, and increased cancer risk |
| X-rays | 0.01 – 10 nm | 30 PHz – 30 EHz | Medical radiography, crystallography | Ionizing; repeated or high exposure can damage tissues and increase cancer risk |
| Gamma rays | < 0.01 nm | > 30 EHz | Astrophysics, nuclear decay | Highly ionizing; very dangerous to humans, causing mutations, burns, and cancers without protection |
Radio waves are the longest in the electromagnetic spectrum, with wavelengths ranging from a few millimeters to several kilometers. They are ubiquitous in our daily lives, used for transmitting information over long distances: radio, television, mobile telephony, Wi-Fi, GPS, and much more.
These waves are generated by antennas and captured by suitable receivers. Their low energy makes them non-ionizing, meaning they do not alter the structure of atoms or molecules. This makes them safe for many applications, including medical and military uses.
Radio waves are classified into several frequency bands: long waves (LF), medium waves (MF), short waves (HF), very high frequencies (VHF), ultra-high frequencies (UHF), and microwaves. Each band has specific uses, depending on range, penetration through obstacles, and data-carrying capacity.
In astronomy, radio telescopes capture radio waves emitted by stars, galaxies, and even the cosmic microwave background, allowing exploration of the universe invisible to the naked eye. These waves are therefore both a pillar of modern technology and a window into the origins of the cosmos.
Microwaves occupy a specific portion of the electromagnetic spectrum, between about 1 millimeter and 30 centimeters in wavelength, corresponding to frequencies from 300 MHz to 300 GHz. They lie between radio waves and infrared waves, giving them unique properties for energy and information transmission.
Due to their higher frequency than conventional radio waves, microwaves can carry large amounts of data over long distances with increased precision. They are used in Wi-Fi, Bluetooth, satellite communications, radar, and of course in microwave ovens, where they vibrate water molecules to heat food quickly.
Because of their higher but still non-ionizing energy, microwaves interact more strongly with matter than radio waves, while remaining safe when used within controlled power ranges. However, their propagation is more sensitive to obstacles and atmospheric conditions, which limits their range without relays.
In astronomy, microwaves are essential for studying the CMB, the fossil radiation from the primordial Universe. Observing this cosmic microwave background, emitted about 380,000 years after the Big Bang, has allowed mapping the first matter inhomogeneities and better understanding the structure and evolution of the Universe.
Infrared waves occupy the part of the electromagnetic spectrum just beyond visible light, with wavelengths between about 700 nanometers and 1 millimeter, corresponding to frequencies from 300 GHz to 430 THz. They are primarily associated with thermal radiation emitted by hot objects, whose temperature is above absolute zero.
These waves are widely used in our daily lives for detection and communication. Remote controls, motion sensors, night vision systems, and some telecommunication technologies exploit the ability of infrared waves to transmit information through the air. They also enable thermography, which visualizes heat distribution on a surface, useful in medicine, industry, and surveillance.
Infrared waves are non-ionizing, but their energy is sufficient to excite molecular vibrations and generate heat. This property is used in radiant heating, industrial infrared ovens, and rapid drying devices for materials.
In astronomy, infrared telescopes observe regions of the Universe often invisible in visible light, such as dust clouds, forming stars, or distant galaxies. These observations help better understand stellar formation processes and the evolution of cosmic structures, providing a complementary view to that obtained by visible and radio waves.
Visible light is the portion of the electromagnetic spectrum perceptible to the human eye, between about 400 nm (violet) and 700 nm (red). It represents a tiny part of the spectrum, but it is the one that has shaped our perception of the world. Each wavelength corresponds to a specific color, and their combination produces white light.
Visible light photons have intermediate energy: sufficient to excite molecules without ionizing them. This is why this light is ideal for observation without alteration, whether in astronomy, biology, or optics. Instruments such as telescopes, microscopes, and cameras exploit this band to capture detailed images.
Visible light also plays a fundamental role in photosynthesis, the process by which plants convert light energy into chemical energy. It is also used in optical fibers, lasers, sensors, and display technologies.
Finally, visible light is at the heart of our visual culture: painting, photography, cinema, design… It is both a scientific tool and a vector of emotion.
N.B.:
Ionize means to remove one or more electrons from an atom or molecule, turning the neutral particle into an ion. This process can change the chemical and biological properties of matter and requires sufficiently energetic radiation, such as ultraviolet, X-rays, or gamma rays.
Ultraviolet (UV) waves are located just beyond visible light in the electromagnetic spectrum, with wavelengths between about 10 nanometers and 400 nanometers, corresponding to frequencies from 750 THz to 30 PHz. Their energy is higher than that of visible light, allowing them to interact strongly with atoms and molecules.
UV rays are widely present in sunlight and are responsible for well-known phenomena on Earth, such as tanning, vitamin D synthesis in the skin, but also skin aging and cancer risks. They are also used in many technological and medical applications: sterilization, UV lamps, spectroscopy, leak detection, and surface treatment.
Unlike radio or microwave waves, UV rays have enough energy to break certain chemical bonds, making them partially ionizing. This property requires adequate protection during their use, while opening the way to precise scientific and industrial applications.
In astronomy, ultraviolet allows the study of hot stars, young galaxies, and star-forming regions. UV telescopes, often placed in space to avoid absorption by Earth's atmosphere, reveal energetic phenomena invisible at other wavelengths, enriching our understanding of the evolution of stars and galaxies.
X-rays are located in the part of the electromagnetic spectrum between ultraviolet and gamma rays, with wavelengths ranging from about 0.01 to 10 nanometers, corresponding to frequencies from 30 PHz to 30 EHz. Their high energy allows them to penetrate many materials opaque to visible light.
These properties make X-rays an essential tool in medicine, where they enable internal imaging of the human body, such as radiographs or computed tomography (CT scans). They are also used in materials science, security (baggage control), and crystallographic analysis to determine the atomic structure of solids.
X-rays are partially ionizing: their energy can remove electrons from atoms, requiring precautions to limit human exposure. This strong interaction capability with matter is also exploited in very precise experimental techniques in physics and chemistry.
In astronomy, X-rays allow observation of extremely energetic phenomena, such as neutron stars, black holes, supernovae, and active galaxies. Since these rays are absorbed by Earth's atmosphere, observation is done from satellites or space telescopes, offering a unique window into the extreme and dynamic Universe.
Gamma rays occupy the most energetic end of the electromagnetic spectrum, with wavelengths less than 0.01 nanometers and frequencies greater than 30 EHz. Their extremely high energy allows them to deeply penetrate matter and cause significant ionization at the atomic and molecular level.
These rays are naturally produced by very energetic astrophysical phenomena, such as supernovae, gamma-ray bursts, black holes, and neutron stars, but they can also be artificially generated for medical or industrial purposes, particularly in radiotherapy for cancer treatment and sterilization of sensitive equipment.
Gamma rays are fully ionizing and require specific protections, such as lead or concrete shields, to limit exposure risks. Their ability to strongly interact with matter makes them a valuable tool for nuclear physics and particle detection experiments.
In astronomy, gamma-ray observation reveals the most violent and energetic events in the Universe. Specialized space telescopes, such as Fermi, map these sources and study the physical mechanisms at work, offering a unique understanding of the extreme processes shaping our cosmos.