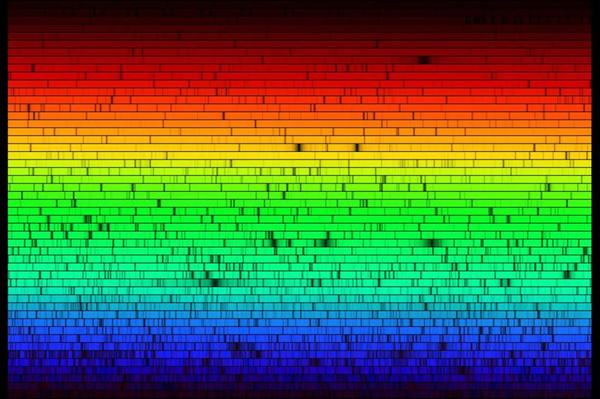
The Visible Light from infrared to ultraviolet is only a small range of vibrations in the electromagnetic spectrum, but it has particular importance because it is the primary information translated by our eyes regarding our environment.
Joseph von Fraunhofer (1787-1826) was the first to notice in 1814, in the visible light of the solar spectrum, the so-called Fraunhofer lines. This German optician and physicist invented the spectroscope in 1815 and was the first to study the diffraction of light using optical gratings (Fraunhofer diffraction).
At that time, the reason for the presence of these Fraunhofer lines in the visible spectrum of light was unknown. It was not until much later, in 1860, that Robert Wilhelm Bunsen (1811-1899) and Gustav Robert Kirchhoff (1824-1887) discovered that the spectral lines of light emitted by an incandescent body constitute a signature that allows this body to be identified. By observing the spectrum of solar light, they recognized several chemical elements present on Earth, including cesium and rubidium. The Sun contains the same chemical elements as the Earth, which was a surprise!
The history of light passes through a series of notable steps in physics: Christian Huygens and his wave theory in 1678, Young's experiment in 1801, Fraunhofer's lines in 1814, Fresnel's diffraction in 1815 (description of the wave nature of light), the solar spectrum of Bunsen and Kirchhoff in 1850, Maxwell's electromagnetism in 1864, the unknown yellow line of helium in 1895, up to Max Planck (1900) and Albert Einstein (1905) for the corpuscular nature of light.
Spectroscopy reveals the nature of the Universe by providing a multitude of unexpected information, often far beyond our expectations. From the observation of color – a simple property of light – we obtain valuable data. For example, blue stars, young and extremely hot, contrast with red stars, older, less massive, and less hot. This principle also applies to galaxies, whose dominant hue (blue or red) allows us to estimate their age.
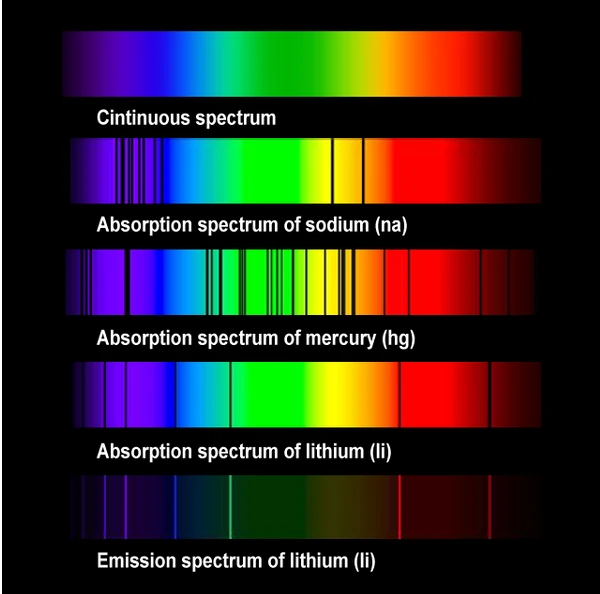
Spectroscopy involves analyzing the spectrum of light, whether visible or not, covering all types of radiation, from radio waves to gamma rays. When we decompose the light coming from a celestial object, we observe emission lines (colored bands on a black background) and absorption lines (dark lines on a colored background). These lines reflect the energy levels of the atoms traversed by the light, thus revealing their chemical composition.
By studying the thickness of these lines, scientists determine the abundance of elements: the more pronounced a line, the greater the quantity of the element present. Spectroscopy also allows us to measure the movement of celestial objects thanks to the Doppler-Fizeau effect (1842).
Thanks to these techniques, astronomers can reconstruct the chemical composition, dynamics, and even the structure of objects as varied as stars, galaxies, quasars, or interstellar clouds. Spectroscopy is thus a window open to the intimacy of the Universe.
When a chemical element is traversed by white light, it absorbs certain wavelengths, revealing dark lines in the diffraction spectrum. These absorption lines, characteristic of the elements, serve as a chemical signature. Thus, by analyzing the white light coming from a star after passing through the ions of its atmosphere, we can determine its chemical composition.
Remarkably, the absorption lines of an element correspond exactly to its emission lines (as shown in the example of lithium in the image). In other words, an element absorbs the same radiations that it can emit, with identical wavelengths.
Rotation of galaxies: By measuring the spectral shifts of stars, we reconstruct the rotation curve of galaxies. Contrary to expectations, the stars on the periphery move as fast as those in the center, revealing the presence of dark matter.
Gamma-ray bursts: These brief flashes of gamma radiation (a few seconds) leave a faint visible glow. Their spectrum reveals the composition of distant stars, offering a glimpse of the young Universe. Thanks to this, we know that the physical laws and chemical elements are uniform throughout the observable Universe.
Cosmic microwave background radiation: Spectroscopy has confirmed that its temperature is 2.7 K, proving that the Universe was hotter in the past.
Finally, this technique is not limited to the visible: it extends from radio waves (low energies) to X-rays and gamma rays (high energies), making it a universal tool for exploring the cosmos.