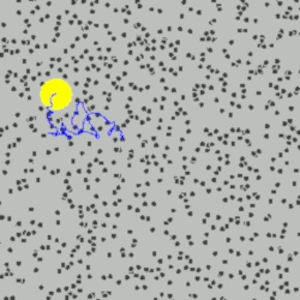
Robert Brown (1773-1858), a Scottish botanist and friend of Charles Darwin (1809 - 1882), discovered in 1827, while observing pollen grains suspended in water under a microscope, that the pollen grains did not remain stationary but moved continuously in an erratic manner for no apparent reason. To confirm that the movement was not specifically related to living pollen, Brown repeated the experiment with inanimate substances, such as fragments of dust and very small inorganic particles. To his great surprise, he found that these inert particles also exhibited the same random motion. Brown documented this phenomenon precisely but did not understand its origin.
For decades, many physicists repeated the experiment with increasingly sophisticated microscopes, eliminating all measurement biases (air currents in the room, currents in the water containing the pollen grains, vibrations, disturbances by light, etc.), yet none found an explanation for this natural agitation.
The kinetic molecular theory finds its roots in the work of several scientists in the 19th century. It was primarily formulated and developed by three major physicists: Rudolf Clausius (1822-1888), James Clerk Maxwell (1831-1879), and Ludwig Boltzmann (1844-1906).
The kinetic molecular theory proposed a model to explain the macroscopic properties of matter in terms of extremely small particles called "molecules" or "corpuscles." Although the concept of the atom was not yet experimentally proven at the time, scientists postulated the existence of tiny invisible entities to account for phenomena such as pressure, temperature, and the gaseous state.
The kinetic theory is based on the idea that all matter is composed of entities in constant motion. At this stage, the existence of atoms was still controversial, but scientists considered the idea of these particles useful for explaining observable phenomena. For example, the pressure exerted by a gas results from the incessant collisions of gas particles against the walls of the container. The more numerous and faster the particles, the higher the pressure. Temperature is interpreted as being proportional to the average kinetic energy of the moving particles. Thus, a hotter gas contains particles that move more quickly.
Without confirmation of the existence of atoms, the kinetic molecular theory remained an abstract model. The idea of moving molecules was accepted by some as a mathematical description, but other scientists remained skeptical, considering these particles as hypothetical entities without direct experimental foundation.
In 1905, the physical reality of atoms and molecules received broader validation, providing a solid foundation for the kinetic molecular theory. The article by Albert Einstein, published on May 11, 1905, titled "On the Movement of Particles Suspended in a Resting Fluid Involved by the Kinetic Molecular Theory of Heat," deals with Brownian motion. Einstein seeks to establish a link between Brownian motion and the kinetic molecular theory, which describes the properties of gases and liquids in terms of molecular movements.
Einstein starts with the idea that in Brownian motion, the suspended dust particles are continuously bombarded by the molecules of the surrounding fluid. The movement of the dust is due to the unbalanced impacts of these molecules, which act randomly but consistently.
Einstein develops an equation that quantitatively describes this random movement. He relates the mean displacement of the particles to time, the temperature of the fluid, the viscosity of the fluid, and the size of the particles (pollen).
The Mathematical Modeling is ⟨x2⟩ = 2kB Tt/6πηr where ⟨x2⟩ is the mean square distance traveled by a particle (pollen) after a time t, �������� is the Boltzmann constant, ���� is the absolute temperature of the fluid, ���� is the viscosity of the fluid, and ���� is the radius of the particle. This equation connects microscopic properties (such as the thermal agitation of molecules) to macroscopic observations (the observable movement of pollen particles or others).
Einstein demonstrates that Brownian motion allows for experimental verification of the existence of atoms and molecules, which were still debated at the time. The movement of the observed particles corresponds exactly to what is predicted by the kinetic molecular theory. Albert Einstein does not directly determine the size of an atom in his article, but he establishes a foundation that will allow for exploration of the atomic nature of matter.
Jean Perrin (1870-1942), a French physicist, conducted a series of experiments in 1908-1909 to verify Einstein's predictions. By measuring the displacements of resin particles suspended in water under a microscope, Perrin observes that the results are consistent with Einstein's calculations, confirming the relationship between particle movement and molecular collisions.
At the microscopic scale, Brownian motion is caused by interactions between invisible molecules (such as water molecules) that move rapidly and chaotically. These molecules interact with the suspended particles, leading to seemingly random movements. At the macroscopic level, Brownian motion is observable to the naked eye (with a microscope) through the behavior of suspended particles. For example, pollen grains can be seen moving erratically in a drop of water.
In summary, Brownian motion acts as a bridge between the microscopic world and the macroscopic world. It illustrates how events at the atomic and molecular scale can translate into measurable and predictable behaviors at larger scales, reinforcing our understanding of physical phenomena at different levels.