Today we approximately know the abundance of chemical elements in the Universe, this value measures the abundance or rarity of elements.
Abundance is a mass ratio or a ratio of molecules present in a given environment relative to other elements. Most expressed abundances are mass ratios. For example, the mass abundance of oxygen in water is about 89%, this is the fraction of the mass of water that is composed of oxygen.
In the observable universe, the mass abundance of hydrogen is 74%. Analyses of the sky by the WMAP satellite indicate that the Universe is composed of 73% dark energy, 23% cold dark matter, and only 4% atoms (chemical elements).
Elements constitute the usual baryonic matter made of protons, neutrons, and electrons, although sometimes in certain regions of the Universe, such as neutron stars, matter is in the form of ions.
Hydrogen is the most abundant element in the universe, followed by helium, oxygen, carbon, neon, nitrogen, magnesium, etc. The abundance of hydrogen and helium, i.e., the lightest elements (atomic number 1 and 2), dominate the other elements which are rare compared to them.
Hydrogen and helium were produced very shortly after the Big Bang during primordial nucleosynthesis. All other elements (the heavier ones) were produced much later in stars during stellar nucleosynthesis. Although hydrogen and helium respectively compose ≈92% and ≈7% of all baryonic matter in the universe, the remaining 1% constitutes considerable masses that have allowed the emergence of life.
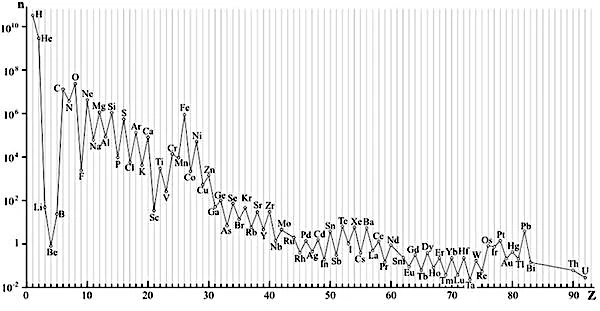
The abundance of elements decreases exponentially with the atomic number (Z). Lithium, Beryllium, and Boron are exceptions that show depletion despite their low atomic number. There is a pronounced abundance peak near iron (Fe). Even Z numbers are more abundant than their odd neighbors, this is what produces this sawtooth effect (even-odd effect) on the curve.
In the primitive solar nebula, hydrogen (H) and helium (He) constitute 99.8% by number of the total elements present. In the remaining 0.2%, we find in order (after H and He) oxygen, carbon, neon, nitrogen, silicon, magnesium, sulfur, argon, iron, sodium, chlorine, aluminum, calcium, etc.
When the rotating primordial cloud cooled, the elements began to condense. The transition from one state of matter to another is called a change of state.
When matter condenses, it changes state, it passes directly from a gaseous state to a solid state without passing through the liquid state, this is what is called in thermodynamics, condensation.
This change occurs under the effect of a modification of volume, temperature, and/or pressure. Thus, near the center of the nebula, we find refractory materials, those that have good heat resistance such as metals and rocks.
Further away, we find volatile materials such as water ice (H2O).
Even further away, there will be molecules of carbon dioxide (CO2), methane (CH4), dinitrogen (N2), and ices. Thus, terrestrial and gas planets "with a solid core" form near the star. Those with a very massive core (two to four times the mass of Earth) have retained the primordial gas of the nebula, these are the gas giants.
| Z | Symbol | Elements | Universe | Sun | Earth |
| 1 | H | Hydrogen | 92 % | 94 % | 0.2 % |
| 2 | He | Helium | 7.1% | 6 % | |
| 8 | O | Oxygen | 0.1 % | 0.06 % | 48.8 % |
| 6 | C | Carbon | 0.06 % | 0.04 % | 0.02 % |
| 10 | Ne | Neon | 0.012 % | 0.004 % | |
| 7 | N | Nitrogen | 0.015 % | 0.007 % | 0.004 % |
| 14 | Si | Silicon | 0.005 % | 0.005 % | 13.8 % |
| 12 | Mg | Magnesium | 0.005 % | 0.004 % | 16.5 % |
| 26 | Fe | Iron | 0.004 % | 0.003 % | 14.3 % |
| 16 | S | Sulfur | 0.002 % | 0.001 % | 3.7 % |
Tables of relative abundance of chemical elements in the Universe, the Sun, and the Earth, the % are approximate. Our organism is composed of 99% CHON (Carbon, Hydrogen, Oxygen, and Nitrogen).