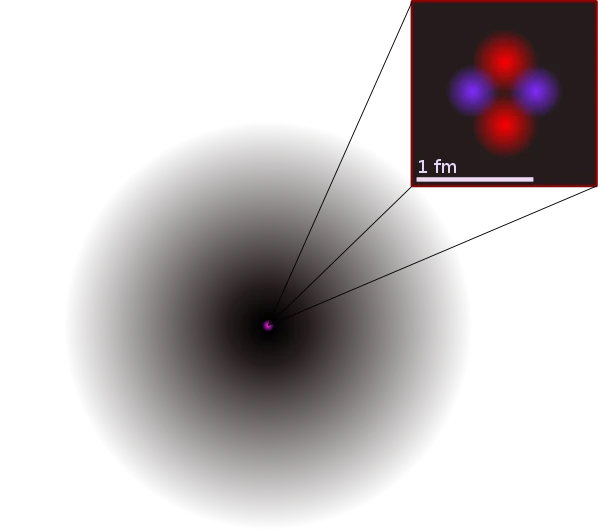
Image description: Representation of the atomic structure of the helium-4 atom. For readability reasons, the image above is not to scale. The atomic nucleus appears in pink in the center and, in shades of gray all around, the electron cloud or atomic orbital. The helium-4 nucleus is schematically enlarged, showing the two protons and two neutrons in red and purple, its size is 1 femtometer or 10^-15 meters. In reality, the nucleus (and the wave function of each of the nucleons) is also spherical, like the electrons of the atom. Image source: public domain.
Everything we see is made up of atoms, many atoms from the Greek word "atomos" (indivisible). A long time ago, in the 4th century BC, the Greek philosophers Leucippus and Democritus hypothesized that all matter is composed of tiny particles in perpetual motion, very solid and eternal. Today we have a slightly more precise idea of the atom because it is not indivisible.
We have known its approximate size since 1811, Amedeo Avogadro estimated the size of atoms to be 10^-10 meters. In 1911, Ernest Rutherford specified the structure of the atom and gave a size to the atomic nucleus of the order of 10^-14 meters.
Regarding the size of atoms, we speak of atomic orbitals, that is, the electron cloud that surrounds the nucleus. This cloud has a theoretical diameter ranging from 62 pm (picometers) for the helium atom to 596 pm for the cesium atom. Nothing is simple in the nature of matter and this tiny distance varies depending on the chemical nature of the surrounding atoms.
Although the nucleus concentrates most of the mass of the atom (99.99%), we also know its mass, for stable atoms, it ranges between 1.674×10^-24 g for hydrogen and 3.953×10^-22 g for uranium.
We also know its composition, inside we see a nucleus and an electron cloud that occupies the entire spatial extent of the atom since it is more than 10,000 times larger than its nucleus. Even more astonishing, we even know the number of atoms in the universe, this number is extraordinarily large, if we had to write it, we would have to write a 1 followed by 72 zeros.
The stability of the atom is not explained by classical physics because in classical physics, the negatively charged corpuscular electron and the positively charged proton raise a paradox.
In classical physics, matter should disappear, annihilate because an electron radiating around a nucleus loses energy (Maxwell's theory) and therefore should fall onto the nucleus. This means that the stability of an atom is incomprehensible within the framework of classical theory.
The scientific geniuses of the 20th century resolved this paradox in 1924, thanks to the wave mechanics of Louis de Broglie (1892-1987) generalized in 1926 by Erwin Schrödinger (1887-1961), Nobel Prize in Physics in 1933 with Paul Dirac (1902-1984) for the wave equation called the Schrödinger equation.
In quantum mechanics, it is not possible to know exactly the value of a parameter without measuring it. The mathematical theory describes a state, not by a precise speed and position pair, but by a wave function (state vector), which allows calculating the probability of finding the particle at a point. Hence the probabilistic nature of quantum mechanics, which predicts that particles are also waves and no longer just material points.
Electrons occupy atomic orbitals in interaction with the nucleus via the electromagnetic force, while nucleons are held together within the nucleus by the nuclear bond.
The electron cloud is stratified into quantized energy levels around the nucleus, defining electron shells and subshells. Nucleons are also distributed in nuclear shells, although a fairly convenient model popularizes the nuclear structure according to the liquid drop model.
Several atoms can establish chemical bonds with each other through their electrons, and in general, the chemical properties of atoms are determined by their electronic configuration, which in turn depends on the number of protons in their nucleus. This number, called the atomic number, defines a chemical element.
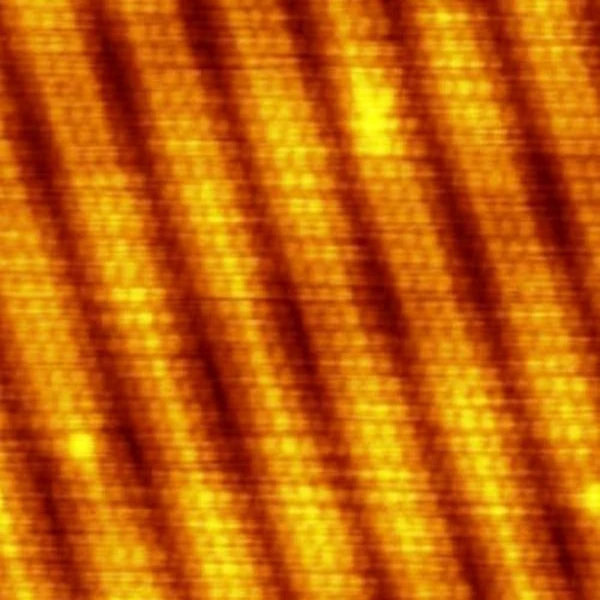
Closest to matter, the surface of a sheet of pure gold (Au 100) is detailed here by a scanning tunneling microscope. The gold atoms visible in this image are regularly spaced on the crystalline structure of gold. This atomic image was made with a low-temperature Omicron STM by Erwin Rossen, Eindhoven University of Technology, in 2006.
Theoretical atomic radius (calculated) of certain atoms (size in picometers (10^-12 meters). The atomic radius is half the distance that separates the nuclei of two contiguous atoms. The values indicated in this table are only indicative.
| Size of atoms in picometers (pm) | |||||||
| (1 pm = 10-12 meter) | |||||||
| size | size | size | size | ||||
| H Hydrogen
: number of electron by energy levels 1 |
53 | Ca Calcium
: number of electron by energy levels 2, 8, 8, 2 |
194 | Y Yttrium
: number of electron by energy levels 2, 8, 18, 9, 2 |
212 | Hf Hafnium
: number of electron by energy levels 2, 8, 18, 32, 10, 2 |
208 |
| He Helium
: number of electron by energy levels 2 |
31 | Sc Scandium
: number of electron by energy levels 2, 8, 9, 2 |
184 | Zr Zirconium
: number of electron by energy levels 2, 8, 18, 10, 2 |
206 | Ta Tantalum
: number of electron by energy levels 2, 8, 18, 32, 11, 2 |
200 |
| Li Lithium
: number of electron by energy levels 2, 1 |
167 | Ti Titanium
: number of electron by energy levels 2, 8, 10, 2 |
176 | Nb Niobium
: number of electron by energy levels 2, 8, 18, 12, 1 |
198 | W Tungsten
: number of electron by energy levels 2, 8, 18, 32, 12, 2 |
193 |
| Be Beryllium
: number of electron by energy levels 2, 2 |
112 | V Vanadium
: number of electron by energy levels 2, 8, 11, 2 |
171 | Mo Molybdenum
: number of electron by energy levels 2, 8, 18, 13, 1 |
190 | Re Rhenium
: number of electron by energy levels 2, 8, 18, 32, 13, 2 |
188 |
| B Boron
: number of electron by energy levels 2, 2 |
87 | Cr Chromium
: number of electron by energy levels 2, 8, 13, 1 |
166 | Tc Technetium
: number of electron by energy levels 2, 8, 18, 13, 2 |
183 | Os Osmium
: number of electron by energy levels 2, 8, 18, 32, 14, 2 |
185 |
| C Carbon
: number of electron by energy levels 2 ,4 |
67 | Mn Manganese
: number of electron by energy levels 2, 8, 13, 2 |
161 | Ru Ruthenium
: number of electron by energy levels 2, 8, 18, 15, 1 |
178 | Ir Iridium
: number of electron by energy levels 2, 8, 18, 32, 15, 2 |
180 |
| N Nitrogen
: number of electron by energy levels 2, 5 |
56 | Fe Iron
: number of electron by energy levels 2, 8, 14, 2 |
156 | Rh Rhodium
: number of electron by energy levels 2, 8, 18, 16, 1 |
173 | Pt Platinium
: number of electron by energy levels 2, 8, 18, 32, 17, 1 |
177 |
| O Oxygen
: number of electron by energy levels 2, 6 |
48 | Co Cobalt
: number of electron by energy levels 2, 8, 15, 2 |
152 | Pd Palladium
: number of electron by energy levels 2, 8, 18, 18 |
169 | Au Gold
: number of electron by energy levels 2, 8, 18, 32, 18, 1 |
174 |
| F Fluorine
: number of electron by energy levels 2, 7 |
42 | Ni Nickel
: number of electron by energy levels 2, 8, 16, 2 or 2, 8, 17, 1 |
149 | Ag Silver
: number of electron by energy levels 2, 8, 18, 18, 1 |
165 | Hg Mercury
: number of electron by energy levels 2, 8, 18, 32, 18, 2 |
171 |
| Ne Neon
: number of electron by energy levels 2, 8 |
38 | Cu Copper
: number of electron by energy levels 2, 8, 18, 1 |
145 | Cd Cadmium
: number of electron by energy levels 2, 8, 18, 18, 2 |
161 | TL Thallium
: number of electron by energy levels 2, 8, 18, 32, 18, 3 |
156 |
| Na Sodium
: number of electron by energy levels 2, 8, 1 |
190 | Zn Zinc
: number of electron by energy levels 2, 8, 18, 2 |
142 | In Indium
: number of electron by energy levels 2, 8, 18, 18, 3 |
156 | Pb Lead
: number of electron by energy levels 2, 8, 18, 32, 18, 4 |
154 |
| Mg Magnesium
: number of electron by energy levels 2, 8, 2 |
145 | Ga Gallium
: number of electron by energy levels 2, 8, 18, 3 |
136 | Sn Tin
: number of electron by energy levels 2, 8, 18, 18, 4 |
145 | Bi Bismuth
: number of electron by energy levels 2, 8, 18, 32, 18, 5 |
143 |
| Al Aluminium
: number of electron by energy levels 2, 8, 3 |
118 | Ge Germanium
: number of electron by energy levels 2, 8, 18, 4 |
125 | Sb Antimony
: number of electron by energy levels 2, 8, 18, 18, 5 |
133 | Po Polonium
: number of electron by energy levels 2, 8, 18, 32, 18, 6 |
135 |
| Si Silicon
: number of electron by energy levels 2, 8, 4 |
111 | As Arsenic
: number of electron by energy levels 2, 8, 18, 5 |
114 | Te Tellurium
: number of electron by energy levels 2, 8, 18, 18, 6 |
123 | At Astatine
: number of electron by energy levels 2, 8, 18, 32, 18, 7 |
127 |
| P Phosphorus
: number of electron by energy levels 2, 8, 5 |
98 | Se Selenium
: number of electron by energy levels 2, 8, 18, 6 |
103 | I Iodine
: number of electron by energy levels 2, 8, 18, 18, 7 |
115 | Rn Radon
: number of electron by energy levels 2, 8, 18, 32, 18, 8 |
120 |
| S Sulfur
: number of electron by energy levels 2, 8, 6 |
88 | Br Bromine
: number of electron by energy levels 2, 8, 18, 7 |
94 | Xe Xenon
: number of electron by energy levels 2, 8, 18, 18, 8 |
108 | ||
| Cl Clorine
: number of electron by energy levels 2, 8, 7 |
79 | Kr Krypton
: number of electron by energy levels 2, 8, 18, 8 |
88 | Cs Caesium
: number of electron by energy levels 2, 8, 18, 18, 8, 1 |
298 | ||
| Ar Argon
: number of electron by energy levels 2, 8, 8 |
71 | Rb Rubidium
: number of electron by energy levels 2, 8, 18, 8, 1 |
265 | Ba Barium
: number of electron by energy levels 2, 8, 18, 18, 8, 2 |
253 | ||
| K Potassium
: number of electron by energy levels 2, 8, 8, 1 |
243 | Sr Strontium
: number of electron by energy levels 2, 8, 18, 8, 2 |
219 | Lu Lutetium
: number of electron by energy levels 2, 8, 18, 32, 9, 2 |
217 | ||