At the beginning of the 20th century, physicists encountered a puzzle: during beta decay, a neutron transforms into a proton while emitting an electron. However, the total measured energy seemed to violate the principle of energy conservation. In 1930, to save this fundamental principle, Wolfgang Pauli (1900-1958) proposed the existence of a neutral, very light, and almost undetectable particle: the neutrino. The latter would be emitted simultaneously with the electron, carrying a portion of the missing energy.
Neutrinos are leptons with a spin of \(\frac{1}{2}\), no electric charge, and an extraordinarily small mass (less than 1 eV/\(c^2\)). Three types (or "flavors") exist: electron neutrino, muon neutrino, and tau neutrino, each associated with a corresponding charged particle. In the case of beta decay, it is the electron neutrino (\(\nu_e\)) that comes into play. These particles pass through matter almost without interacting: billions of neutrinos from the Sun or terrestrial nuclear reactions pass through us every second without leaving a detectable trace. Their interaction is exclusively of the weak type, making their detection extraordinarily difficult.
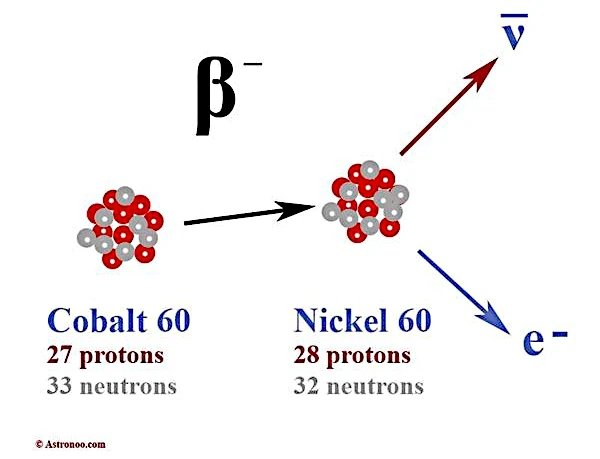
In a typical beta decay, a neutron transforms according to the scheme:
\(n \rightarrow p + e^{-} + \bar{\nu}_e\)
where \(n\) is the neutron, \(p\) the proton, \(e^{-}\) the electron, and \(\bar{\nu}_e\) the electron antineutrino. The introduction of the neutrino restores the laws of conservation of energy, momentum, and angular momentum. For example, the continuous spectrum of the energy of the electrons emitted in beta decay can only be explained if another particle carries a random fraction of this energy, which is precisely what the neutrino does.
The experimental proof of the existence of the neutrino came in 1956 thanks to the experiment of Frederick Reines (1918-1998) and Clyde Cowan (1919-1974) at the Savannah River Plant, by detecting the antineutrino emitted by nuclear reactors. Their method is based on the inverse interaction:
\(\bar{\nu}_e + p \rightarrow n + e^{+}\)
The observation of the positron (\(e^{+}\)) and the secondary neutron provides an indirect signature of the passage of the neutrino. Since then, giant detectors like Super-Kamiokande or IceCube continue to track these messenger particles, which inform us about both terrestrial nuclear processes and supernovae or the core of the Sun.
The neutrino is a particle with a spin of \(\frac{1}{2}\), like all leptons. But unlike other fermions (elementary particles), it has a fascinating particularity: it does not behave in the same way as its antiparticle under the fundamental symmetries of physics. This asymmetry is manifested in particular in the violation of parity (\(P\)) observed in weak interactions.
In 1957, the historic experiment of the Sino-American physicist, Chien-Shiung Wu (1912-1997) showed that the electrons from beta decay are emitted preferentially in a direction opposite to the spin of the nucleus, thus demonstrating that nature distinguishes the right from the left, a major break with the dogma of universal symmetry. This result means that the neutrinos produced in beta decays are always of left-handed chirality, while antineutrinos are of right-handed chirality.
This property is expressed in terms of helicity: the neutrino propagates with its spin oriented antiparallel to its direction of movement (\(h = -1\)). On the other hand, if the neutrino had zero mass, its helicity would be invariant under Lorentz transformation. The fact that neutrinos can oscillate between flavors implies that they have mass, and thus do not travel exactly at the speed of light. This reopens the possibility, in theory, of transforming a neutrino into an antineutrino by inversion of an inertial frame, which would have profound implications for CP violation and the structure of the extended standard model.
Thus, the spin of the neutrino is not a simple quantum property: it reveals a deep asymmetry of nature, visible in the fact that only left-handed chirality is involved in the weak interaction. In other words, the spin of the neutrino shows that nature does not treat the left and the right in the same way: only the "left" version of the neutrino participates in the weak interaction.
The discovery of the phenomenon of neutrino oscillation (their ability to change flavor during their propagation) has revolutionized particle physics by implying a non-zero mass. This has forced the modification of the standard model and suggests paths towards physics beyond it. Neutrinos could even play a role in the matter/antimatter asymmetry of the universe or explain part of the missing mass in the cosmos.
Initially conceived as an ad hoc correction to an energy anomaly, Pauli's particle has proven to be a central actor in modern physics. Invisible but omnipresent, the neutrino is the silent messenger of beta radioactivity, carrying with it precious clues about the most fundamental laws that govern matter and the universe.
| Neutrino | Associated Particle | Symbol | Mass < (eV/c²) | Flavor Type | Interaction |
|---|---|---|---|---|---|
| Electron Neutrino | Electron | \(\nu_e\) | < 1.1 | Electronic | Weak |
| Muon Neutrino | Muon | \(\nu_\mu\) | < 0.17 | Muonic | Weak |
| Tau Neutrino | Tau | \(\nu_\tau\) | < 18.2 | Tauic | Weak |
Sources: PDG (Particle Data Group, 2024), Super-Kamiokande, IceCube.