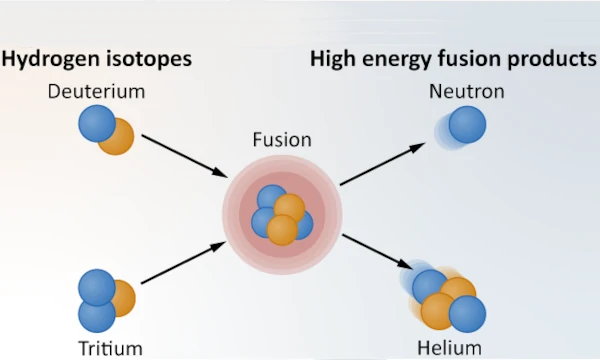
Nuclear fusion is one of the most energetic processes in the universe, playing a central role in energy production in the cores of stars. Thus, the fusion of atomic nuclei, that is to say their union to form a heavier nucleus, requires enormous amounts of energy to overcome certain fundamental forces that act at microscopic scales. What are these forces that need to be overcome to fuse atomic nuclei?
The strong nuclear force is the most powerful of the four fundamental forces. The range of the strong interaction is about 10^-15 meters, which is the size of a nucleon (about 1 femtometer). This force is extremely powerful at short distances and is responsible for the cohesion of nucleons within the nucleus. It is attractive and surpasses the electrostatic repulsion between protons when they are very close to each other. Without this force, protons, which repel each other due to their positive charge, could not remain together in the nucleus. However, this force practically does not act beyond a few femtometers.
Thus, to stick two protons together, they must be brought extremely close to each other. Once the protons are sufficiently close (at a distance of about 1 femtometer), the strong nuclear force comes into play. This force is attractive and works to hold the nucleons (protons and neutrons) together in the nucleus.
N.B.:
The strong interaction is equal to 1 (this is the reference force). Its coupling constant is about one hundred times greater than that of the electromagnetic interaction, one million times greater than that of the weak interaction, and 10^38 times greater than that of gravity.
The Coulomb force is a fundamental force of nature that describes the interaction between electric charges (electrons, protons, ions, etc.). It applies only to objects carrying electric charges, whether positive or negative. It is one of the pillars of electromagnetism.
Between protons, the electrostatic repulsive force is omnipresent due to their positive charges. This force acts over greater distances (well beyond the few femtometers of the strong nuclear force) and creates what is called the Coulomb barrier. This barrier is the main energy obstacle that prevents charged nuclei from fusing at low energy. It is equal to 10^−2 (relative to the strong nuclear force).
Thus, when two atomic nuclei come closer, their potential interaction energy increases with the Coulomb repulsion, then passes through a maximum that must be overcome to achieve nuclear fusion.
Protons, which are positively charged, exert an electrostatic repulsive force against each other, known as the Coulomb force. For fusion to occur, this electrostatic repulsive force must be overcome.
The strong nuclear force acts at very short range, typically on the order of 1 femtometer. Thus, the nuclei must be very close for this force to act. This requires considerable energy to bring the nuclei close enough for the strong nuclear force to take over and allow fusion.
To overcome these two forces, nuclear fusion requires very high speeds, and thus extremely high temperatures, to provide the necessary kinetic energy. These conditions are found at the core of stars, where temperatures of several tens of millions of degrees Celsius are reached due to the enormous pressures at the heart of these stars. At such high temperatures, matter exists in a state of plasma, where electrons are separated from the nuclei, allowing for greater freedom of movement for the nuclei themselves.