The photoelectric effect is one of the most fundamental discoveries of modern physics. This phenomenon, first observed in the late 19th century, profoundly transformed our understanding of light and matter.
Understanding the photoelectric effect marked a decisive turning point in the development of quantum mechanics. It validated the hypothesis of light quanta and paved the way for a new era in physics.
At the beginning of the 20th century, physicists faced a perplexing paradox that challenged the very foundations of classical optics. According to the wave theory established by James Clerk Maxwell (1831-1879), the energy carried by a light wave was proportional to its intensity.
Physicists therefore expected that:
However, the meticulous experiments of Philipp Lenard (1862-1947) and other researchers revealed completely unexpected behavior:
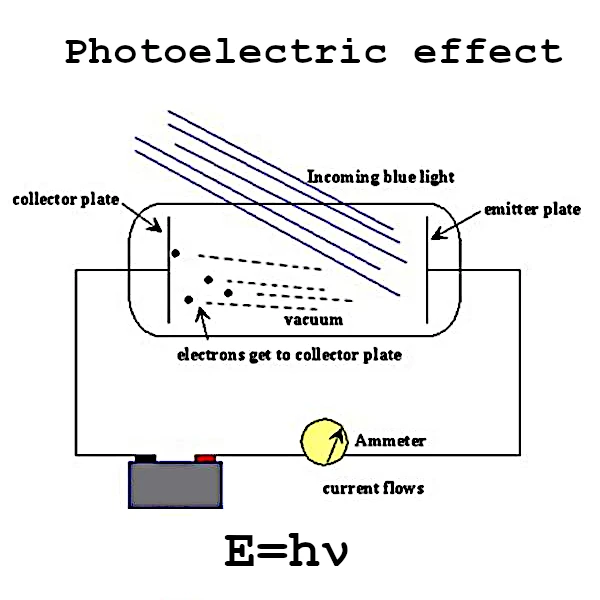
This paradox was particularly evident with red light: even at very high intensity, it failed to dislodge even a single electron from certain metals like zinc. On the other hand, violet light, even at very low intensity, immediately caused electron emission.
This fundamental contradiction between classical theory and experimental observations created a theoretical deadlock that only a new conception of light could resolve.
In 1905, Albert Einstein (1879-1955) proposed a revolutionary explanation that would earn him the Nobel Prize in 1921. He suggested that light is composed of photons, each possessing a well-defined energy: \( E = h \nu \) where \( h \) is the Planck constant and \( \nu \) is the frequency of the light.
N.B.:
The Planck constant is extremely small (\( h = 6.62607015 \times 10^{-34} \mathrm{J \cdot s} \)), which explains why quantum effects are not observable at our macroscopic scale but become dominant at the atomic and subatomic scale.
The photoelectric effect obeys three fundamental laws:
The maximum energy of the emitted electrons is expressed as: \( E_{max} = h\nu - \phi \) where \( \phi \) represents the work function of the material.
N.B.:
The photoelectric effect was crucial in establishing the quantum nature of light. Unlike classical waves, energy is exchanged in discrete packets, photons, whose energy depends only on their frequency.
| Phenomenon | Nature of Light | Energy of Electrons | Effect Threshold |
|---|---|---|---|
| Photoelectric effect | Particulate (photons) | Depends on frequency | Minimum frequency required |
| Classical thermal effect | Wave-like | Depends on intensity | No threshold |
Source: Nobel Prize in Physics 1921 - Albert Einstein and Encyclopædia Britannica - Photoelectric Effect.
The photoelectric effect, a quantum phenomenon deciphered by Albert Einstein (1879-1955), is now the fundamental principle of many essential technologies in our modern society.
Photovoltaic cells are the best-known application. When photons from sunlight strike semiconductors (usually silicon), they dislodge electrons, thus creating a direct electric current.
CCD and CMOS sensors are found in all modern cameras. Each pixel contains photodiodes that generate electrons proportional to the received light intensity.
Detection systems exploit the precise sensitivity of photodetectors to different types of radiation (security gates, smoke detectors, rangefinders, etc.).
Scientific research uses extremely sensitive photoelectric detectors for matter analysis and cosmic exploration (photomultipliers, spectrometers, space telescopes, etc.).
The medical field also benefits from applications of the photoelectric effect for diagnosis and treatment (endoscopic cameras, Positron Emission Tomography scanners, photodetectors for blood analysis, etc.).
Economic impact: The market for technologies based on the photoelectric effect represents several hundred billion euros annually, with particularly strong growth in the photovoltaic and digital imaging sectors.