Carbon monoxide (CO) and carbon dioxide (CO₂) are two simple compounds of carbon and oxygen, but their physiological properties differ radically. While CO is a highly toxic gas at very low concentrations, CO₂ is a gas constantly produced by our metabolism, tolerated within certain limits. However, the only structural difference between these two molecules is the presence of an additional oxygen atom in CO₂.
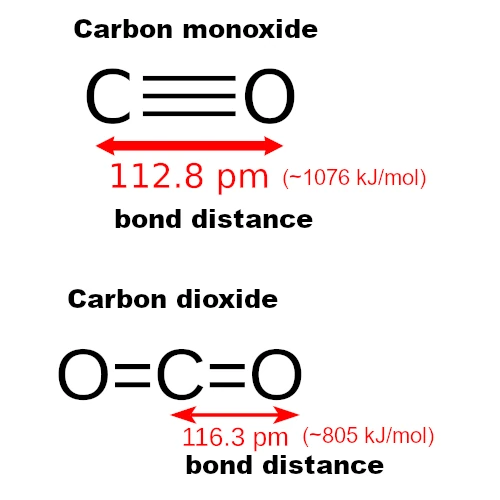
CO contains only two atoms (carbon and oxygen), so its geometry is linear (a single axis of alignment). The atoms are aligned in a straight line: C–≡–O (two atoms aligned).
CO₂, on the other hand, is also linear but symmetrical and non-polar, with two double bonds: O=C=O (three atoms in a straight line).
This structural difference alters chemical reactivity, interaction with biomolecules, and thus physiological toxicity.
The toxicity of CO comes from its very strong affinity for hemoglobin, approximately 230 to 300 times greater than that of oxygen. Once bound to hemoglobin to form carboxyhemoglobin (HbCO), CO prevents O₂ from binding and alters its release into tissues, leading to severe hypoxia. At 0.1% (1000 ppm) of CO in the air, a person can lose consciousness within minutes.
On the other hand, CO₂ does not bind to hemoglobin in the same way. It is produced in the mitochondria during cellular respiration, partially transported in the form of bicarbonate \( \text{HCO₃⁻} \), and exhaled by the lungs. This gas even plays a key role in regulating blood pH.
Although it is a metabolic waste product, carbon dioxide is not inherently toxic in low concentrations. However, when it exceeds 2-3%, it disrupts the acid-base balance by forming carbonic acid \( \text{CO₂ + H₂O ⇌ H₂CO₃ ⇌ H⁺ + HCO₃⁻}\), leading to respiratory acidosis. It is only above 5-8% CO₂ in the air that serious neurological effects and vital risks are observed.
CO₂ becomes toxic to the human body at 2-3%, with serious and potentially fatal effects above 5% in the air breathed.
An adult at rest exhales about 200 mL of CO₂ per minute. Over 8 hours of sleep, each person generates about 90–100 L of CO₂ per night.
Consider a room of 15 m² with a ceiling height of 2.5 m, the total volume = 37,500 L. The CO₂ exhaled by one person represents: \(\frac{100\ \text{L}}{37\,500\ \text{L}} = 0{,}0027 = 0{,}27\%\) Addition of 0.27% CO₂ in the room over a full night, without ventilation.
Outdoor air contains ~0.04% CO₂. In a closed room with 2 people, the level can rise between 0.2% and 0.5% (2000–5000 ppm). This level remains below pathological thresholds (the first symptoms of hypercapnia begin at around 2-3%).
Even with several people, CO₂ remains well below toxic thresholds during a typical night, unless the room is extremely small, completely airtight, and with no air leakage (which is rare).
An apparently minor modification in molecular structure – such as the addition or removal of a single atom – can lead to profoundly different, even opposite, biochemical effects.
This shows how much chemical properties emerge from fine molecular structure. And it underscores why predicting chemical and biological interactions is complex: it depends not only on the elements present but on their spatial arrangement, polarity, symmetry, molecular orbitals…