Our Galaxy contains about 200 billion stars, and statistics tell us it contains 2 trillion planets. This seems sufficient to say that life has certainly found many places among the habitable zones of these billions of stars to develop. However, so many favorable conditions must be met that the possibilities are severely restricted.
The first essential condition is the presence of liquid water. Water is abundant in the material Universe, which consists of 74% hydrogen, 24% helium, 1% oxygen, and all other elements combined represent only 1% of ordinary matter.
Scientists believe that liquid water is vital due to its role in biochemical reactions. It is even considered an essential element for a viable ecosystem because it greatly facilitates the transport of materials necessary for biochemical activity.
A planet must therefore have liquid water and keep it long enough, for billions of years, to have a chance of sustaining life. No one knows how life appears; the transition from inanimate to animate is still a mystery, but life as we observe it on our planet is based on carbon chemistry in solution in liquid water.
If life exists elsewhere, it must be based on carbon chemistry. Life did not take the path of silicon, which is much more abundant on Earth than carbon.
Specialists have tried to imagine life in a chemistry other than carbon, and all say it is much more complicated because carbon (C) is the essential component of organic compounds. It is the basis of a multitude of compounds and associates very well with other atoms, particularly hydrogen, oxygen, nitrogen, phosphorus, and sulfur.
Since the water molecule (H2O) is present everywhere in the Universe, all planets must have it. The difficulty will be to keep the water on the surface in a liquid state for billions of years, and for this, the habitable planet must meet many conditions favorable to its stability.
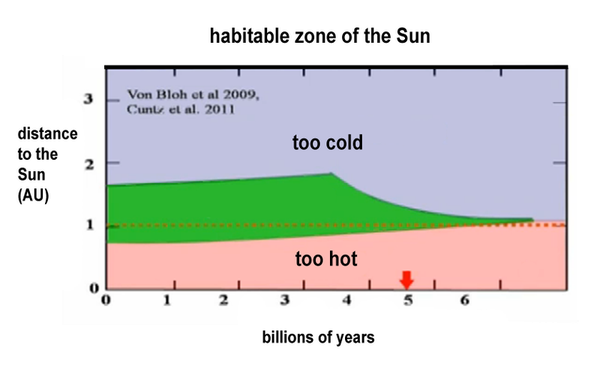
Therefore, the planet must remain in orbit in a habitable zone.
Numerical models show that if Earth is moved 12% away from the Sun, it will then receive only 79% of the Sun's heat. At this point, the climate spirals out of control, and Earth is very rapidly covered in ice within a few decades. Yet Earth has always been habitable despite the evolution of the Sun's heat.
At the beginning, the Sun was 25% less bright than today, yet there has always been liquid water on Earth's surface with an average climate warmer than today. This is the paradox of the Faint Young Sun.
This old enigma from 1972 was raised by astronomers Carl Sagan (1934-1996) and George Mullen. It happened when evidence of liquid water and bacterial life was found in the geological layers from the early formation of Earth. Sagan and Mullen suggested at the time that a greenhouse effect three times stronger than today must have existed thanks to ammonium and methane.
Thanks to this primordial greenhouse effect, Earth could retain the weak heat emitted by the young Sun. As the Sun grew brighter, the carbonate-silicate cycle took over to stabilize Earth's climate.
If the planet can stabilize its climate, the upper limit of the habitable zone moves back to 1.6 AU. The inner limit of the habitable zone has been calculated by computer models to be 5% (0.95 AU). At this point, closer to the Sun, the climate system spirals out of control. The temperature increases, the greenhouse effect intensifies, and the oceans evaporate. The bonds of water molecules are broken in the atmosphere, and the very light hydrogen disappears into space. Little by little, the planet loses all its water.
It is therefore between 0.95 and 1.65 AU that Earth can keep liquid water on its surface. It must remain in this zone for a very long time to allow the evolution of life. It is thanks to the carbonate-silicate cycle and plate tectonics that Earth has been able to retain its atmosphere.
The Carbonate-Silicate Cycle plays a crucial role in regulating Earth's climate over long periods. This cycle begins when atmospheric CO2 dissolves in rainwater, forming carbonic acid (H2CO3). The products of this weathering are then transported to the oceans, where marine organisms use them to make calcium carbonate (CaCO3) shells. Upon death, these organisms accumulate on the ocean floor. Plate tectonics then recycles the CO2 in subduction zones, releasing it as gas into the atmosphere through volcanic eruptions. Without this volcanic release, atmospheric CO2 would be depleted in about 400,000 years. The cycle continues when volcanic CO2 is again dissolved by rainwater, which transports it to the ocean depths. This process is essential for the functioning of Earth's "climate machine."
In summary, to keep water in a liquid state, a living planet with intense geological activity is needed. However, the phenomenon of plate tectonics is absent on the other planets in the solar system. It seems that if the planet is too small, like Mars, it cannot have plate tectonics, but if the planet is larger (super-Earth), convection is less efficient, and there will only be one large plate. Yet Venus, which is the same size as Earth, does not benefit from plate tectonics!
N.B.:
The circumstellar habitable zone, also called the ecosphere, is a theoretical region in the shape of a circular tube around a star where the surface temperature of orbiting planets allows the presence of liquid water. Although this zone could potentially harbor life, the conditions necessary for it to develop are so numerous that the probabilities remain very low.
Another essential condition for maintaining water in a liquid state concerns the astronomical characteristics of the star around which a planet orbits. These characteristics are limited by the fact that 60% of stars are binary systems, which is not conducive to the emergence of life, as the orbits of planets in these systems are often irregular and chaotic. For a planet to be habitable, its orbital eccentricity must be low, close to 0 (circular orbit). Earth's eccentricity, which is 0.01, is ideal. In contrast, observations of exoplanets reveal an average eccentricity of 0.29, which is considerable. High eccentricity makes the planetary orbit unstable, exposing the planet to gravitational perturbations from other planets (work by Jacques Lascar), which also affects temperature stability. Few of the observed solar systems offer nearly circular orbits like those in our solar system.
Another crucial condition for retaining liquid water is the mass of the star. Stars have masses ranging from about 1/100th to 100 times that of the Sun. Stars more massive than the Sun, between 1.2 and 1.5 solar masses, emit too many ultraviolet rays, which is not favorable for the emergence of life. Moreover, their lifespan is too short to allow the development of life.
Stars smaller than the Sun emit a significant amount of X-rays and particles harmful to life. About 75% of stars have a mass of about 0.5 solar masses. These stars emit little light, bringing the habitable zone closer to the star. Due to this proximity, potentially habitable planets synchronize their rotation with their star through tidal effects, resulting in one frozen face and one scorched face. Although a "temperate region" may exist at the boundary, this situation is not ideal for maintaining water in a liquid state. Furthermore, at this distance, these planets do not have a magnetic field, as synchronization prevents the differential rotation of the planetary core, exposing them even more to solar radiation.
In summary, stars with 0.9 to 1.2 solar masses are the most conducive to the emergence of life. Computer simulations are not surprising, as they model the ideal conditions corresponding to our solar system. However, they help researchers better understand the complex conditions necessary for the emergence of life.
N.B.:
Eccentricity defines the shape of an elliptical orbit; it varies between 0 and 1. 0 for circular orbits. High eccentricity decreases the smallest axis (perihelion) and increases the largest axis (aphelion) but does not modify the major axis.
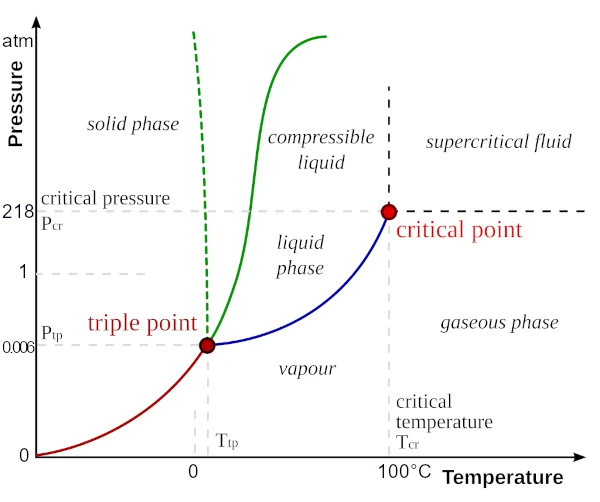
Pure water exists in a single phase (solid, liquid, or gaseous) for a specific pressure and temperature. However, at the triple point, the three phases coexist simultaneously at a precise temperature and pressure. A pressure-temperature pair can correspond to a phase transition, i.e., a change of state, such as melting (solid to liquid), solidification (liquid to solid), sublimation (solid to gas), deposition (gas to solid), vaporization (liquid to gas), or condensation (gas to liquid). Beyond the critical point, water enters a fluid phase, where it is both gaseous and liquid, at a pressure of 218 atmospheres and a temperature of 374°C.