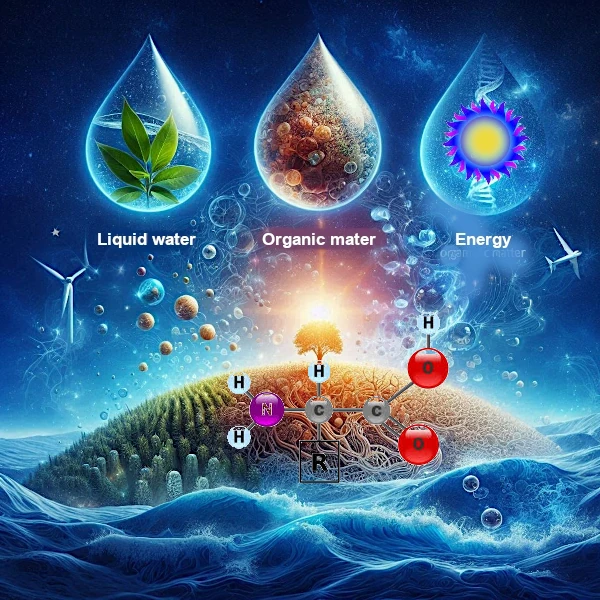
The emergence of life begins with the assembly of the first complex organic molecules, such as amino acids, which are the fundamental building blocks of proteins. For these molecules to form and establish strong chemical bonds, three components are essential: liquid water, organic matter, and a source of energy.
These amino acids must coexist under specific conditions, which are found on Earth but also potentially elsewhere, such as on certain meteorites.
How do the three components of life interact to allow the formation of the first amino acids, and why is their simultaneous presence so rare in the Universe?
N.B.:
An amino acid is characterized by the presence of two functional groups: an amine group (-NH2) and a carboxyl group (-COOH), both attached to a central carbon atom, called the alpha carbon (Cα). This alpha carbon is also linked to a hydrogen atom and a side chain (R), which varies depending on the specific amino acid.
Liquid water is often considered the cradle of life.
Indeed, liquid water plays a central role in the formation of amino acids. It acts as a universal solvent, allowing organic molecules to dissolve, move, and interact with each other. Water also facilitates chemical reactions by stabilizing reaction intermediates and allowing the formation of peptide bonds, essential for linking amino acids together.
On Earth, aquatic environments, such as hydrothermal vents, likely served as cradles for these reactions. However, liquid water is rare in the Universe, as it requires very specific temperature and pressure conditions.
Organic matter, rich in carbon, hydrogen, nitrogen, and oxygen, forms the basis of amino acids.
These chemical elements are abundant in the Universe, but their assembly into complex molecules such as amino acids, for example NH2-CH(R1)-COOH, requires specific conditions.
The formation of amino acids, whether through chemical methods such as the Strecker reaction or through processes simulating the primitive conditions of Earth like the Miller-Urey experiment, requires the presence of certain starting compounds (organic precursors) and an appropriate reaction environment. These conditions allow the necessary chemical reactions to synthesize amino acids, which are essential for the construction of proteins.
The formation of amino acids could also occur in space.
N.B.:
Organic matter is primarily composed of carbon, often in combination with other elements such as hydrogen, oxygen, nitrogen, sulfur, and phosphorus.
Energy is necessary to activate the chemical reactions that transform organic precursors into amino acids.
On Earth, this energy can come from various sources, such as ultraviolet light from the Sun, lightning, or geothermal activity.
In space, energy sources include cosmic rays, stellar luminosity, gravitational energy, tidal forces, or meteorite impacts. For example, experiments have shown that amino acids can form under simulated space conditions, under the influence of radiation or thermal shocks.
Although the basic ingredients of amino acids are widespread in the Universe, the three simultaneous conditions necessary for their formation are rare.
Liquid water, in particular, is difficult to maintain outside the habitable zone of a star.
Organic matter must be sufficiently concentrated and exposed to an appropriate energy source for chemical reactions to occur.
But beyond these conditions, long-term stability is also required. Indeed, for molecular assemblies to continue to evolve and find the path to life, the three elements – liquid water, organic matter, and energy – must coexist stably for millions, even billions of years. This temporal stability is crucial for allowing chemical reactions to become more complex and give rise to more elaborate biological structures.
Liquid water, organic matter, and energy are the three key components that allow the assembly of the first amino acids, the fundamental building blocks of life. Although their simultaneous presence on Earth allowed the emergence of life, this combination over the long term is rare in the Universe. However, the discovery of amino acids on meteorites suggests that these molecules could also form in extraterrestrial environments, opening the possibility that life could start elsewhere than on Earth.