In physics, a fundamental constant is a quantity whose value is fixed and universal, that is to say valid everywhere in the universe. Constants are essential to our understanding of the universe because they are the basis of many laws and theories in physics. They are also used to define the units of measurement of the International System of Units (SI).
A constant cannot be deduced from physical laws and must be measured experimentally, as precisely as possible.
Fundamental constants play a crucial role in physics because they come into play in many fundamental equations, and therefore in our understanding of the world.
In other words, these fundamental constants are intrinsically linked to the SI base units because they define the fundamental properties of the universe and enable the precise measurement and understanding of physical phenomena at different scales.
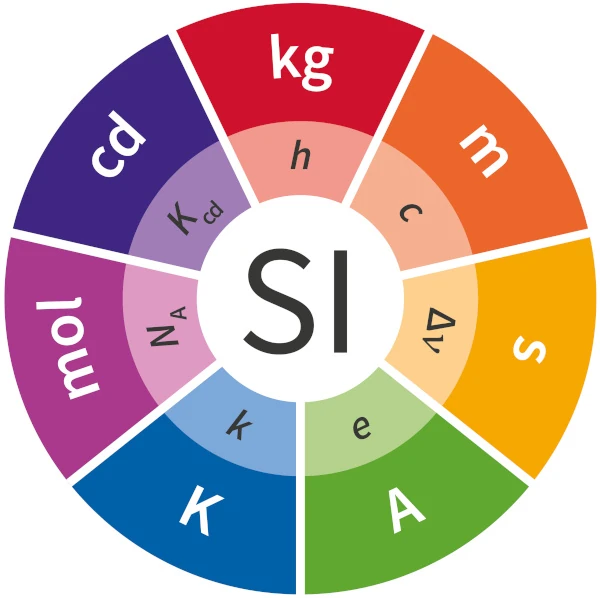
The seven fundamental constants essential to define the seven base units of the International System of Units (SI) are:
1. Frequency of the hyperfine transition of the ground state of the Cesium 133 atom, represented by (ΔνCs), is equal to 9,192,631,770 Hz.
This constant defines the second(s).
A Cesium 133 atom passes from the hyperfine level F=3 to the hyperfine level F=4 by emitting a photon with a frequency of 9,192,631,770 Hz. Its value has not changed significantly since it was first measured in 1955. In other words, one second is defined as the duration of 9,192,631,770 cycles of this transition. This means that the second is an extremely precise unit of time. Current accuracy is about 1 second in 300 million years.
2. Speed of light in vacuum, represented by (c), is equal to 299,792,458 m/s.
This constant defines the meter (m).
The accuracy of the speed of light in a vacuum is currently 1 part in 10^15. This means that the measured value is 299,792,458 meters per second with an uncertainty of 0.000,000,000,000,001 meters per second. It is one of the most precisely measured physical constants. The current accuracy of the speed of light translates to a meter accuracy of about 3 × 10^(-10), or about 30 nanometers.
3. Planck's constant, represented by (h), is equal to 6.626 070 15 × 10^(-34) kg m^2 s^-1 (or J s).
This constant defines the kilogram (kg).
This means that the kilogram is now defined as the mass of an object that has a kinetic energy of 6.626070 15 × 10^(-34) J when moving at a speed of 1 meter per second. The definition based on Planck's constant is independent of any particular physical object. The relative precision is about 2 × 10^(-8), which means that the mass of a kilogram is known with an uncertainty of about 20 micrograms.
4. Elementary charge, represented by (e), is equal to 1.602 176 634 × 10^(-19) C.
This constant defines the ampere (A).
The elementary charge is the electric charge carried by a proton or an electron. The coulomb (C) is the unit of electric charge. It is defined as the amount of electricity carried by a current of one ampere for one second. In other words, one ampere is equal to one coulomb per second 1 A = 1 C/s. The accuracy of elementary charge is currently about 2.5 × 10^(-8). This means that the value of e is known with a relative uncertainty of about 25 parts per billion.
5. Boltzmann's constant, represented by (k), is equal to 1.380 649 × 10^(-23) J/K.
This constant defines the kelvin (K).
Boltzmann's constant is essential in statistical thermodynamics. It is related to the base unit of joule per kelvin (J/K) in the SI, because it relates thermal energy to absolute temperature. The Kelvin is the thermodynamic unit of temperature, while the Joule is the unit of energy. The accuracy of Kelvin is approximately 2 × 10^(-8), or approximately 20 microkelvins.
6. Avogadro's number, represented by (NA), is equal to 6.022 140 76 × 10^(23) mol^(-1).
This constant defines the mole (mol).
Avogadro's number represents the number of elementary entities (atoms or molecules) in a mole of matter, which is crucial for calculations in chemistry and materials physics. So, a mole is a quantity that contains exactly NA elementary entities, whether atoms, ions, molecules or particles. The relative precision of this value is about 2.5 × 10^(-8), which means that NA is known with an uncertainty of about 15 parts per billion.
7. Candela spectral light intensity, represented by (I_c), is equal to 683 lumens per watt.
This constant defines the candela (cd).
The spectral light intensity of the candela is exactly equal to 683 lumens per watt for a wavelength of 540 × 10^12 hertz (green light). It is a fundamental concept in photometry that allows us to quantify and compare human perception of light at different wavelengths. The relative uncertainty in the realization of the candela is currently approximately 2 × 10^(-8). This means that the value of the candela is known with an uncertainty of approximately 20 parts per billion.