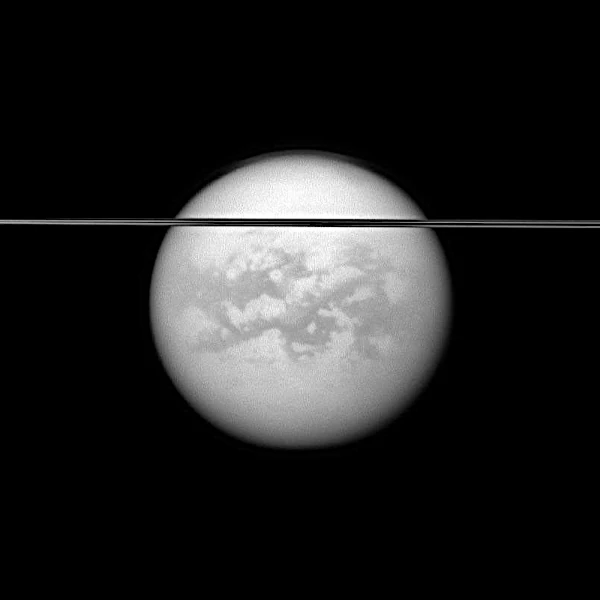
Discovered in 1655 by Christiaan Huygens (1629-1695), Titan is Saturn’s largest moon and the second largest in the Solar System after Ganymede, Jupiter’s largest moon. With an average radius of \(R = 2575\ \mathrm{km}\) and an average density of \(\rho = 1.88\ \mathrm{g.cm^{-3}}\), Titan is a complex world composed of a mixture of ices and silicate rocks. But its most fascinating feature is its dense atmosphere, composed of 98% nitrogen and 1.4% methane, with traces of more complex hydrocarbons.
Surface pressure reaches \(1.47\ \mathrm{bar}\), higher than Earth’s. This atmosphere, maintained by photochemical processes, hosts a true methane cycle: evaporation, clouds, rain, and liquid hydrocarbon lakes, mainly in polar regions. Methane seas, such as Kraken Mare and Ligeia Mare, cover several hundred thousand km².
Radar images from the Cassini-Huygens mission (2004–2017) revealed a complex terrain: equatorial dunes, dry riverbeds, and fossil deltas. The Huygens probe, which landed in 2005, confirmed the presence of a surface rich in tholins — organic molecules produced by the photodissociation of methane under solar UV radiation.
Beneath a thick ice crust several tens of kilometers deep, Titan may hide a subsurface ocean about 100 km deep. Tidal models based on Cassini measurements indicate slight orbital deformation consistent with a fluid mantle. This ocean, rich in salts and ammonia, could maintain conditions favorable to complex prebiotic chemistry.
| Moon | Parent planet | Radius (km) | Density (g/cm³) | Atmosphere | Average temperature (K) | Albedo | Feature |
|---|---|---|---|---|---|---|---|
| Ganymede | Jupiter | 2634 | 1.94 | Very thin (O₂) | ≈110 | 0.43 | Largest moon in the Solar System, own magnetic field |
| Titan | Saturn | 2575 | 1.88 | Dense (N₂, CH₄) | ≈94 | 0.22 | Methane cycle, hydrocarbon seas |
| Callisto | Jupiter | 2410 | 1.83 | Very thin (CO₂, O₂) | ≈134 | 0.17 | Ancient, heavily cratered surface |
| Io | Jupiter | 1821 | 3.53 | Very thin (SO₂) | ≈110 | 0.63 | Intense volcanic activity |
| Europa | Jupiter | 1561 | 3.01 | Very thin (O₂) | ≈102 | 0.67 | Likely subsurface ocean |
| Moon | Earth | 1737 | 3.34 | None | ≈220 | 0.12 | Earth’s only natural satellite |
| Triton | Neptune | 1353 | 2.06 | Very thin (N₂, CH₄) | ≈38 | 0.76 | Active geysers, retrograde rotation |
| Rhea | Saturn | 764 | 1.23 | None | ≈99 | 0.70 | Craters, possible tenuous ring |
Sources: ESA – Cassini-Huygens Mission, NASA – Saturn Moons, JPL Solar System Exploration.
On worlds like Titan, where temperatures are around 94 K and water is frozen, organic liquids such as methane and ethane could serve as alternative biological solvents. Unlike water, these hydrocarbons are non-polar, profoundly affecting chemical interactions and the structure of molecules capable of forming membranes. Chemical reactions would be much slower, but the low polarity could favor the formation of stable structures like azotosomes, able to encapsulate complex reactions in closed compartments.
Note:
Azotosomes are hypothetical membranes made of nitrogen-based molecules stable at very low temperatures, such as those present in liquid methane on Titan. They could play a role similar to terrestrial lipid membranes, encapsulating chemical reactions and facilitating prebiotic chemistry in cryogenic environments.
For prebiotic chemistry to develop under these conditions, efficient catalysts and sufficiently flexible molecules would be needed to maintain structural stability at cryogenic temperatures. Hypothetical membranes could protect organic molecules from radiation and allow local concentration of rare reactants. Although fully speculative, this exotic form of life would offer a radically different perspective on terrestrial biology, with metabolic cycles adapted to extreme conditions and chemical interactions dominated by Van der Waals forces rather than hydrogen bonds.
Note:
Van der Waals forces are weak attractive interactions between molecules, resulting from induced or permanent temporary dipoles. Although much weaker than covalent or ionic bonds, they play an essential role in stabilizing molecular structures, the cohesion of liquids and membranes, and even in cryogenic environments where azotosomes might exist on Titan.
In summary, life based on methane or ethane is not excluded by physics and chemistry, but it would require extreme adaptations and unprecedented molecular structures, illustrating the potential diversity of the chemistry of life in the universe.