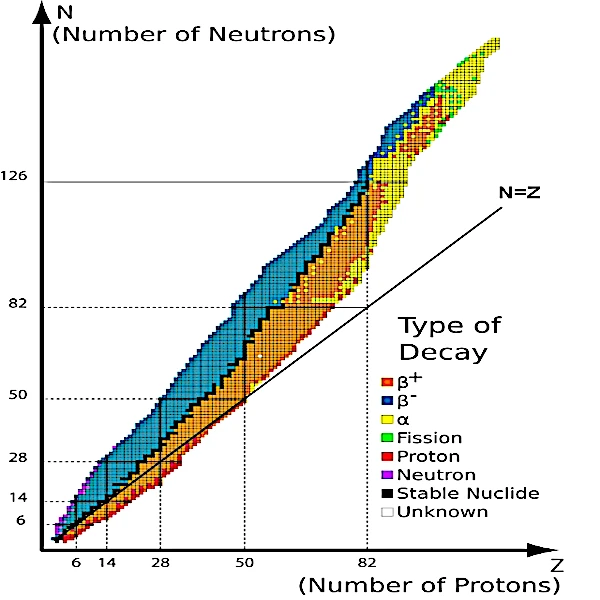
Image description: The stability valley of isotopes by type of radioactive decay.
It can be observed that for Z<20, all stable nuclei lie on the N=Z bisector.
For Z>20, all stable nuclei lie above the N=Z line in the stability valley. Nuclei must incorporate more and more neutrons relative to protons as they grow.
For Z>83, no stable nuclei exist despite the observable limit of the number of protons, estimated to be around Z=126.
Unstable nuclei undergo a cascade of radioactive decays to ultimately join the river of stable matter flowing at the bottom of the valley.
In nature, all matter is concentrated in the mass energy of nuclei 100,000 times smaller than an atom, but thousands of times heavier than all their electrons.
In matter, many atomic nuclei are stable, and their state remains indefinitely the same. An isotope is stable when it has a harmonious number of protons and neutrons. On the other hand, many nuclei are unstable because they have too many protons or neutrons, or both. Physicists have identified just under 300 stable isotopes and nearly 3,000 unstable ones. If nuclei are unstable, it is due to the Coulomb barrier, which sets up a competition between the electrostatic repulsion force between protons and the nuclear attractive force between neutrons and protons. That is why nuclei must incorporate more and more neutrons as they grow.
All matter nuclei seek a frugal energy stability. Thus, to return to a stable state, they must transform by expelling energy in the form of mass or radiation (E=mc^2). This is what we call radioactivity.
This natural radioactivity phenomenon is at work everywhere in matter, both in minerals and in our food (the European Community has set limits for radioactivity in food) and even in our bodies (due to the presence of carbon-14 and potassium-40).
• When all known isotopes are plotted on a graph (image above) by their number of protons (Z) and their number of neutrons (N), it is observed that all stable isotopes (black dots) are clustered around a line. This line is at the bottom of a valley called the "stability valley."
Unstable nuclei are distributed on the flanks of the valley on either side of this black line representing the river of stable matter flowing at the bottom of the valley. The more unstable the nuclei, the higher they are in the valley. Thus, in this valley, the shortest path to stability is to descend to the bottom of the valley.
• Nuclei on the left flank of the valley (in blue in the image), with excess neutrons compared to their number of protons, find stability through a cascade of β- decays with the emission of electrons and neutrinos, allowing them to gradually descend the valley slopes.
• Nuclei on the right flank of the valley (in orange), with excess protons, find stability through a cascade of β+ decays with the emission of positrons and neutrinos.
• Nuclei on the small left ridge of the valley (in purple) at the outer edge of the blue zone, find stability through neutron emission; the nucleus retains the same atomic number (Z) but its atomic mass decreases.
• Nuclei located on the small right ridge of the valley (in red) at the outer edge of the orange zone, find stability through proton emission; the atomic number (Z) and the atomic mass of the nucleus decrease.
• On the side of very heavy nuclei, fission occurs. Nuclei beyond the line of stable isotopes (in light green) find stability through the splitting of the nucleus into two lighter nuclei with the emission of one or more neutrons.
• High atomic mass nuclei (in yellow) undergo α decay often accompanied by the emission of high-energy photons or gamma rays. If the slope is too steep, β decays intersperse with α decays. A cascade of radioactive decays is necessary to reach the bottom of the valley.
• Finally, particularly stable nuclei have a certain number of nucleons (2, 8, 20, 28, 50, 82, and 126) which correspond to the shell model of the atomic nucleus (quantized energy levels based on the Pauli exclusion principle). These so-called magic numbers are marked along the steps of the black curve. Nuclei with both a magic number of protons and neutrons and a number of protons equal to the number of neutrons are called doubly magic because they are very stable.
This is the case for lead-208, which consists of 82 protons and 126 neutrons, and calcium-48, consisting of 20 protons and 28 neutrons.
• Discontinuities in the black curve in neutrons (19, 21, 35, 39, 45, 84, 115, and 123) and protons (43 and 61) correspond to cases where there are no stable nuclei with these quantities of nucleons.
The stability valley contains about 3,000 observed nuclei, stable, unstable, and very unstable. But we do not know the stability limits (drip lines) of the nuclei. For protons (maximum number of protons), it is relatively known through the Mendeleev table. For neutrons (maximum number of neutrons), the stability limits are only known for the first elements, from hydrogen to oxygen-15. For example, for Z=8, the maximum number of neutrons is 16, making oxygen-24 the heaviest possible isotope of oxygen. The complete extent of the stability valley remains unknown, and it seems that what remains to be discovered is enormous.
N.B.:
The nucleus of an atom is made up of protons and neutrons. Atoms of the same chemical element have the same number of protons but can have a different number of neutrons; these are isotopes. Stable atoms do not undergo radioactive decay.