Quantum state superposition is a fundamental principle of quantum mechanics, where a particle can exist simultaneously in multiple possible states until measured. This phenomenon, theorized by Erwin Schrödinger (1887‑1961) and other early 20th-century pioneers, highlights the inherently probabilistic and non-deterministic nature of subatomic systems.
In a superposition state, each component of the probability wave contributes to the particle’s overall dynamics, leading to interference and entanglement effects. These effects, invisible at the macroscopic scale, are crucial for understanding collective quantum behavior and form the theoretical basis for emerging technologies such as quantum computing, ultra-precise sensors, and quantum cryptography.
Superposition also illustrates the concept of wave function, which describes the transition of a quantum system from multiple states to a single observable state upon measurement, emphasizing the fundamental difference between microscopic reality and macroscopic experience.
In a superposition state, each component of the probability wave influences the overall quantum dynamics. Observation or measurement triggers a wave function collapse, forcing the system into a single measurable state. This mechanism illustrates the inherently probabilistic nature of quantum mechanics and the fundamental difference with classical systems.
Superposition lies at the core of many quantum phenomena, notably entanglement, where multiple particles become nonlocally correlated, and the interference patterns observed at the microscopic scale. It also enables quantum coherence, essential for technologies like quantum computing, ultra-precise atomic clocks, and highly sensitive magnetic and gravitational sensors.
The fundamental implications of superposition go beyond experiments, raising deep questions about reality, measurement, and observation in the subatomic world.
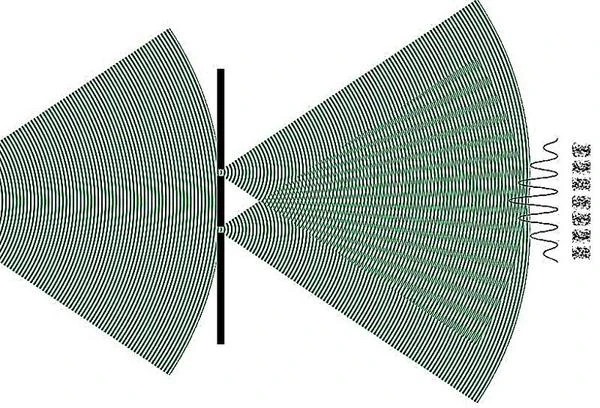
Quantum state superpositions are evidenced through sophisticated interferometry experiments using photons, electrons, neutrons, or ultracold trapped atoms cooled by lasers. These experiments directly reveal interference patterns, showing the simultaneous coexistence of multiple quantum states.
Technologies exploiting superposition are central to quantum computing, where qubits can simultaneously represent 0 and 1, exponentially increasing computational power for certain tasks. They also include ultra-precise atomic clocks, which use quantum coherence to measure time with unparalleled accuracy, and quantum cryptography, ensuring unbreakable communication through entanglement and superposition.
These experimental methods and technological applications show that quantum superposition is not merely theoretical but a fundamental resource for advanced technologies and exploration of quantum physics limits.
| State type | Main characteristic | Example | Applications |
|---|---|---|---|
| Classical state | Determined, measurable | Position of a ball, velocity of a car | Newtonian physics, mechanical engineering |
| Quantum state | Superposition of multiple states | Electron spin, electron position in an atom | Interferometry, quantum sensors, quantum computing |
| Quantum entanglement | Instant correlation between separated particles | Entangled photon pairs, entangled electrons | Quantum cryptography, quantum teleportation, quantum networks |
| Coherent state | Minimal quantum fluctuation superposition | Laser, quantum harmonic oscillator | Quantum optics, quantum communication, metrology |
| Pure state | Complete knowledge of the wave function | Ground state of an isolated atom | Quantum simulation, quantum computing, fundamental experiments |
| Mixed state | Statistical mixture of pure states | Atomic system at finite temperature | Quantum thermodynamics, decoherence, quantum sensors |
| Stationary state | No temporal change in probability | Energy levels of a hydrogen atom | Spectroscopy, atomic clocks |
| Excited state | Energy higher than the ground state | Electron in a higher atomic level | Luminescence, lasers, spectroscopy |
| Ground state | Minimal possible energy | Electron in the lowest level | Atomic stability, reference for atomic clocks |
Quantum superposition illustrates the uniqueness of subatomic physics, where classical rules no longer apply and reality exists in a potential state until measured. This concept is central to modern research in quantum computing and advanced quantum technologies.