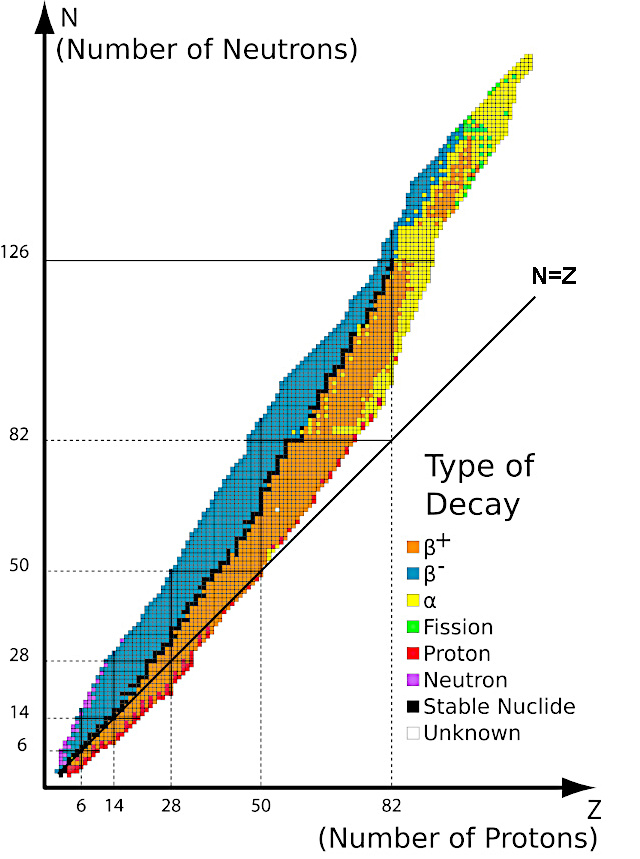
Image: The isotope stability valley by type of radioactive decay.
- In black: stable nuclei, they do not undergo radioactive decay and do not emit radiation.
- In orange, blue and yellow: unstable nuclei which disintegrate either by radioactivity β either by radioactivity α to go down into the valley.
- In red and purple: unstable nuclei which have too many protons or neutrons emit either a proton or a neutron to descend into the valley.
- In pale green: unstable nuclei which have too many protons and neutrons must fission to descend into the valley.
We can notice that for Z<20, all of the stable nuclei are located on the bisector N=Z.
For Z>20, all of the stable nuclei are located above the line N=Z in the stability valley. The nuclei must integrate more and more neutrons relative to protons as they grow.
For Z>83, there are no more stable nuclei despite the observable limit of the number of protons which is estimated at approximately Z=126.
The unstable nuclei undergo a cascade of radioactive disintegration to ultimately flow into the river of stable material flowing at the bottom of the valley.
Stars are centers of nuclear fusion where chemical elements are created from the lightest to the heaviest. Lighter elements are combined by nuclear fusion to form heavier elements. The energy released during this reaction is used to maintain the temperature and pressure of the star's core.
The amount of energy released during fusion depends on the mass of the atomic nuclei that are merging. However, the heavier the nuclei, the less energy is released.
There is a nuclear instability barrier from iron.
Iron is the chemical element with the most stable nucleus. The fusion of two iron nuclei does not release energy, but on the contrary absorbs it. This is why nuclear fusion stops at iron. That is, beyond iron, the formation of heavier elements would require energy input instead of releasing energy, unlike the fusion of elements lighter than iron.
This is explained by the valley of stability of atomic nuclei and the nuclear processes involved.
When lighter elements fuse together in the core of a star, it releases energy because the mass of the resulting core is slightly less than the sum of the masses of the original cores. Nuclei lighter than iron can release energy by fusing to reach a more stable configuration.
From Z=20, the nuclei, as they grow, must integrate more and more neutrons compared to protons. Thus, beyond iron (atomic number Z=26), nuclear fusion requires absorbing energy rather than releasing it. That is, for nuclei heavier than iron, fusion leads to less stable nuclei and requires energy input rather than energy release.
This is where the nuclear instability barrier is located.
Outside the core of aging stars, in the outer layers, processes occur responsible for the formation of elements heavier than iron.
Slow neutron capture, also called s-process, is one of two major neutron capture processes that contribute to the formation of elements heavier than iron.
In the red giant phase, the star undergoes nuclear reactions, releasing energy. Light nuclei present in the outer layer of the star slowly capture neutrons, forming unstable nuclei. Some of the nuclei formed by neutron capture then undergo beta decay, where a neutron is converted to a proton, thereby increasing the atomic number of the nucleus.
This process repeats several times, leading to the formation of heavier elements in a progressive manner. These elements extend beyond iron on the periodic table.
The elements formed by the s-process are ejected into space when the star expels its outer layers as a planetary nebula.
The s-process is characterized by slow neutron captures, hence its name. It is responsible for the production of many stable heavy elements such as silver, barium and lead.
The other process is a rapid neutron capture process, called r-process. It occurs during supernova explosions, or during a merger of two neutron stars. This process releases a large number of neutrons. By rapidly capturing neutrons, the nuclei of atoms become unstable. A certain number of unstable nuclei undergo beta decay, where a neutron transforms into a proton and emits an electron and an antineutrino. This increases the atomic number, turning the element into a heavier one.
The r process is responsible for the production of elements such as gold, platinum, and uranium in the universe.