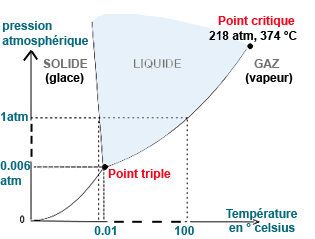
Image: Diagram of change of state or phase transition of pure water.
At the triple point, the 3 phases (solid, liquid, gaseous) coexist at a given temperature and pressure.
At the critical point (218 atm, 314°C), water reaches the supercritical phase between liquid and gas.
The supercritical state of water is a special state that lies beyond its traditional states of solid, liquid and vapor. The supercritical state does not exist in everyday life.
In the supercritical state, the molecules of a substance have high kinetic energy, but they are not far enough apart to form a gas. Therefore, supercritical fluids have unique properties that distinguish them from both liquids and gases.
At high temperatures and pressures, water can reach a point where the distinctions between liquid and gaseous phases become blurred. It is then in a state called "supercritical" where it has unique physico-chemical properties.
Thanks to its intermediate density between that of liquid water and that of vapour, supercritical water has a high dissolution capacity for many chemical compounds. It can move more easily through porous materials as if it were a gas, but with better dissolving ability due to its higher density.
The temperature at which water becomes supercritical depends on the pressure applied. For pure water, the supercritical state is usually reached at temperatures above 374 degrees Celsius (647 Kelvin) and pressures above 220 bar (or 22 MPa).
Supercritical water is used in various fields of scientific research and in industry. For example, it is used for the extraction of compounds from plants, for decaffeinating coffee, or in cleaning, purification and waste treatment applications.
In our oceans with a certain depth, the water reaches the critical pressure (22 MPa) at a depth of 2240 meters. The average depth of the oceans is 3 to 5 km, so a significant part of the ocean floor exceeds this critical pressure. But the critical temperature is 374°C, a temperature well above the average temperature of the ocean floor (close to 0°C). However, at the ocean ridges, molten basalts arrive. The average depth of the ridges is 2700 m, below the “critical depth”. These basalts are at a very high temperature, over 1100°C, so we can very well imagine that a part close to the active ridges is in the supercritical state of water.
The surface of the planet Venus is mainly composed of carbon dioxide (CO2). However, the supercritical state of carbon dioxide is reached at a temperature of 31°C (critical temperature) with a pressure of 7.3 MPa (critical pressure). The surface temperature of Venus is close to 400°C for a pressure of about 90 times Earth's atmospheric pressure. The lower atmosphere of Venus is therefore in a supercritical state.
The gas giant planets composed mainly of hydrogen and helium certainly have supercritical states of matter in their layers.
Hydrogen and helium also have very low critical points, meaning they can be easily brought to the supercritical state at relatively low temperatures and pressures. Hydrogen and helium become supercritical when they have a temperature above 33.2 K (-240.2°C) and a pressure above 22.07 MPa.
In the supercritical state, hydrogen and helium have a density similar to that of liquid water, but they have a viscosity and surface tension similar to that of a gas.
Beyond the solar system, compositions of exoplanets have been modeled and it could be that certain planets (55 Cancri e and Gliese 876 d) have an ocean of water in a supercritical state.