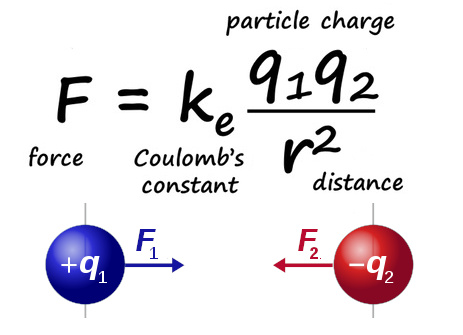
Image: Named after Charles-Augustin de Coulomb, Coulomb's law (F=kqq/r²) is an equation which represents the attractive or repulsive electric force (F) of two point charges (q).
The Coulomb equation is one of the fundamental laws of electrostatics describing the electrical interaction between electric charges. This equation is named after the French physicist Charles-Augustin de Coulomb (1736-1806), who formulated it in the 18th century.
Coulomb's equation is expressed mathematically as follows: F = k q₁ q₂ / r²
- F is the electrostatic force between two point electric charges q₁ and q₂.
- k is Coulomb's constant (8.9875×109 N m2/C2), which depends on the system of units used and vacuum permittivity (capacity of an empty space to polarize in response to an applied electric field).
- r is the distance between the two charges.
The electrostatic force acts on positive and negative electric charges, and it can be attractive (between opposite charges) or repulsive (between charges of the same sign).
This force is described by Coulomb's law. Electric charge is expressed in coulombs (C) in the international system of units (SI). Electrons carry a negative elementary charge of approximately -1.602 x 10-19 C, while protons carry a positive charge equal in absolute value.
The electrostatic force decreases with the square of the distance between charges. This means that the further apart the charges are, the faster the force decreases. Conversely, the closer the loads are, the more the force increases. This explains the repulsion between protons which are positively charged particles. According to Coulomb's law, the electrostatic force between two protons is directly proportional to the product of their positive charges (q1 and q2) and inversely proportional to the square of the distance separating them (r2). As this distance (r) is very small, of the order of a femtometer (1 femtometer = 1×10-15 m), the electrostatic force is enormous.
Coulomb's equation is valid for immobile charges in the context of static electricity. If the charges are moving, one would have to take into account the effects of special relativity (magnetism) and use Maxwell's equations of electromagnetism.
The electrostatic force is a fundamental force of nature, and it is responsible for many electrical phenomena observed in everyday life.
Static electricity is an electrical phenomenon that occurs when stationary electrical charges build up on the surface of an insulating object. Electrical charges are formed when objects are rubbed together, creating sparks or feelings of electric shock.
Lightning is a very powerful electrical natural phenomenon that occurs when there is an electrical discharge between clouds charged with static electricity and the ground or between the clouds themselves.
The Aurora Borealis and Australis are spectacular light displays in the polar regions caused by charged particles from the solar wind colliding with charged particles from Earth's atmosphere.
St. Elmo's fires are luminous phenomena that sometimes occur on sharp objects such as ship masts or the tops of trees during thunderstorms. They are caused by the buildup of electrical charges in the air around these objects.
Ball lightning is floating electric light spheres that can appear during thunderstorms and move erratically.
Atmospheric static electricity is also responsible for the presence of an ion layer in the Earth's ionosphere, which plays an important role in radio communications and electromagnetic wave refraction phenomena.