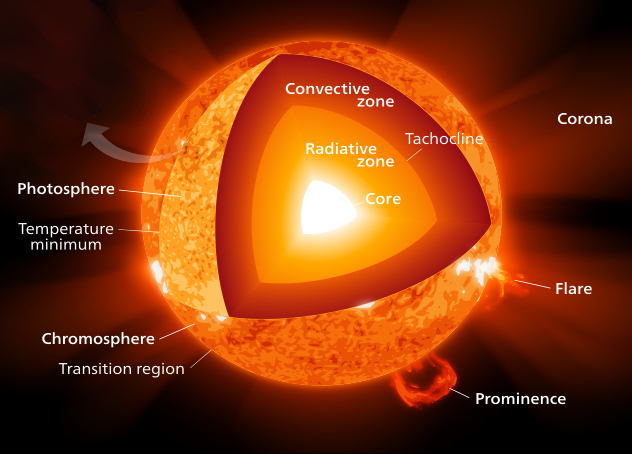
Image: The heat produced in the core of the Sun is transported to the surface by convection and radiation.
Convection transfers gas atoms from the core at 15 million Kelvin to the surface (photosphere) at 6000 K through the different layers of the Sun. This movement of hot gas is visible on the surface of the Sun in the form of convection cells.
Radiant heat transfer occurs when gamma photons created in the core interact with electrons from hot gas atoms being moved to the surface. These repeated interactions eventually transfer heat to the Sun's surface, where it is ultimately radiated out to space as visible light and infrared heat, with an effective temperature of 5,500 to 6,000 Kelvin.
Heat and temperature are two different concepts in thermodynamics but closely related.
Temperature is a measure of the molecular agitation of an object, while heat is a form of energy that is transferred between two bodies with different temperatures. Thus, the temperature is an intensive quantity which depends only on the thermodynamic state of an object, while the heat is an extensive quantity which depends on the quantity of matter and the variation of temperature.
In other words, temperature measures the average thermal agitation of molecules in an object, while heat measures the amount of energy transferred between two objects with different temperatures.
Temperature is a physical quantity that measures the molecular agitation of a body or a system.
This thermal agitation corresponds to the random movement of molecules which is directly related to the temperature of the body or of the system. Put more simply, this quantity can be measured in degrees Celsius (°C) or in kelvins (K) using a thermometer.
The equation of the thermal agitation temperature of a body corresponds to the average kinetic energy of the particles.
E = ½m v² where E is the average kinetic energy of a particle, m its mass and v its average speed.
According to the kinetic theory of gases, the equation below shows that the velocity of particles in a gas increases with temperature.
v = sqrt((3*k*T)/m) where T is the temperature of the gas, k is the Boltzmann constant (1.38 x 10^-23 joules per kelvin) and m is the mass of the particle.
The equation below shows that the average kinetic energy of the particles of a gas is proportional to the absolute temperature T. Thus, the thermal agitation temperature is directly related to the system temperature.
E = (3/2) * k * T where E is the kinetic energy, T is the gas temperature, k is the Boltzmann constant.
Heat is a form of energy that is transferred from one system to another due to a difference in temperature.
Heat is measured in joules (J) or calories (cal), one calorie is approximately 4.184 joules. It is transferred either by conduction (direct contact), or convection (contact by the movement of a fluid) or by radiation (transfer of heat in the form of electromagnetic waves).
For heat transfer by conduction, say through a flat plate of thickness L, Fourier's law gives the amount of heat Q that is transferred per unit time through the plate:
Q = -k * A * (dT/dx) where k is the thermal conductivity of the plate, A is its area, dT/dx is the temperature gradient along the plate, and the minus sign indicates that the heat is transferred from the hot region to the cold region.
For convective heat transfer between two fluids, Newton's local heat transfer equation gives the amount of heat Q that is transferred per unit time and area:
Q = h * A * (T1 - T2) where h is the heat transfer coefficient, A is the contact surface between the fluids, T1 and T2 are the temperatures of the two fluids, and the minus sign is omitted because the heat is transferred from T1 (hottest fluid) to T2 (coldest fluid).
For the transfer of heat by radiation between two bodies, the Stefan-Boltzmann law gives the quantity of heat Q which is emitted per unit area of a black body at temperature T1 and which is received per unit area of a other black body at temperature T2:
Q = σ * A * (T1^4 - T2^4) where σ is the Stefan-Boltzmann constant (σ = 5.670 374 419 x 10^-8 W m^-2 K^-4) and A is the area of both bodies.
This law assumes that bodies are perfect black bodies, that is, they absorb all radiation that reaches them and emit radiation that follows Planck's law, which describes the emission spectrum electromagnetic radiation emitted by a hot body.
N.B.: These equations are not always applicable in practice, because the heat transfer conditions can be complex and often depend on many parameters, such as the geometry of the bodies, the thermal properties of the materials, the flow velocity of the fluids, etc.