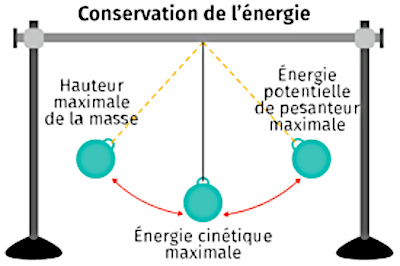
The Schrödinger equation, named after the Austrian physicist Erwin Schrödinger (1887-1961), is one of the foundations of quantum mechanics. It describes the temporal evolution of the quantum states of physical systems. This equation is deterministic, that is to say that it makes it possible to predict the state of a system at any time from its initial state.
It is written: iħ∂ψ/∂t = Hψ
where ψ is the wave function of the system, H is the Hamiltonian operator which represents the total energy of the system, ħ is the reduced Planck constant, and t is the time.
The wave function ψ is a complex mathematical function that describes the quantum state of a system. It contains all the information about the physical properties of the system, such as position, momentum, energy, spin, etc. The wave function ψ can be used to calculate the probability of finding a particle in a given region of space.
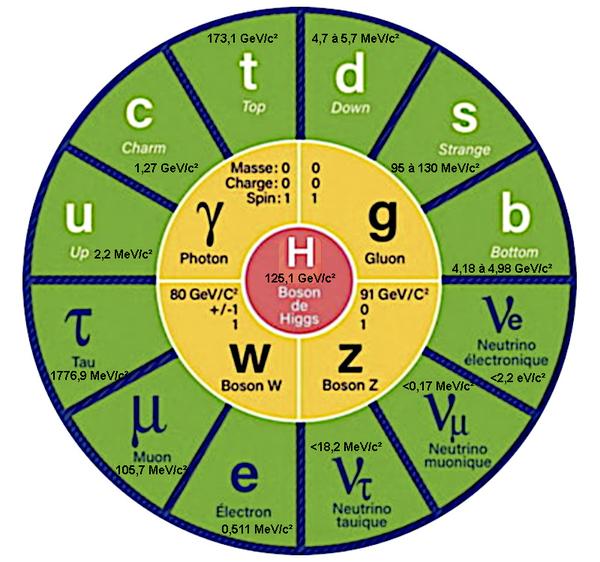
The Hamiltonian operator H represents the total energy of the system. It is defined as the sum of the kinetic and potential energies of all the particles in the system. Kinetic energy represents energy due to the motion of particles while potential energy represents energy due to interactions between particles. | | Planck's reduced constant ħ is a fundamental physical constant that has a value of 1.054571817×10-34 joule-second. It is used to quantify the energy levels of quantum systems and to describe the wave-particle duality of quantum particles.
Schrödinger's equation is a partial differential equation, which means that it describes the evolution of the wave function ψ as a function of time and position in space. This equation is linear, which means that if two solutions of the equation are added, their sum is also a solution.
What is iħ∂ψ/∂t = Hψ for?
The Schrödinger equation is used to study many quantum systems, such as atoms, molecules, subatomic particles, solids, liquids, etc.
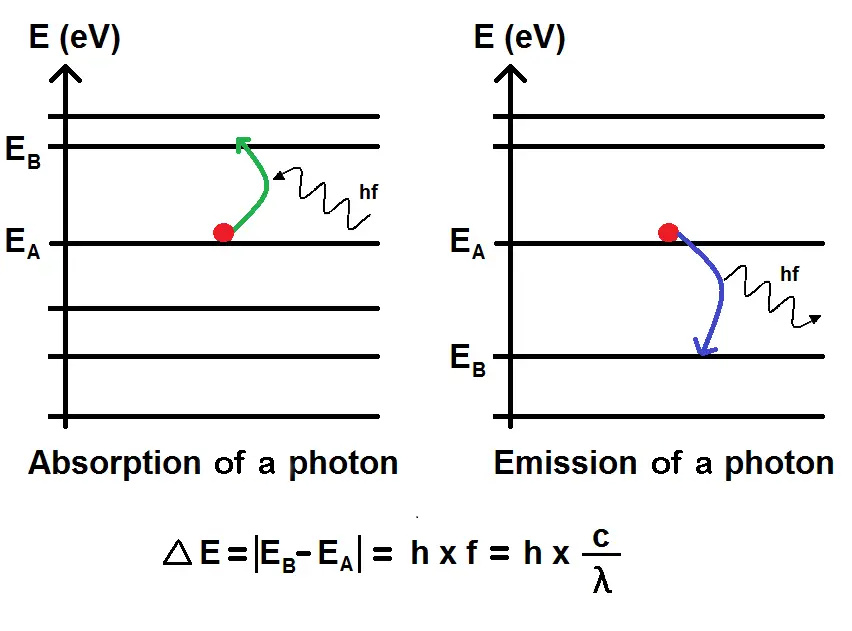
It makes it possible to predict the physical properties of these systems, such as energy levels, wave functions, probability densities, emission spectra, etc.
However, the Schrödinger equation does not directly give the physical observables, like position or momentum, but only the probability of measuring them. | |  Image: Schrödinger is known for his thought experiment on Schrödinger's cat concept. This experiment helped explain the complex ideas of quantum physics because at the time there was debate about the nature of quantum reality. Schrödinger's cat concept illustrates the principle of quantum superposition and the notion of measurement. The experiment involves a cat in a box with a device that can kill the cat with a certain probability, based on the decay of an atom. According to quantum theory, before observing the box, the cat is in a state of superposition where it is both dead and alive. |
 Automatic translation
Automatic translation






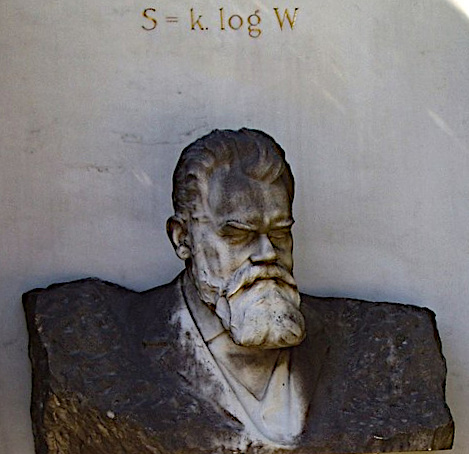
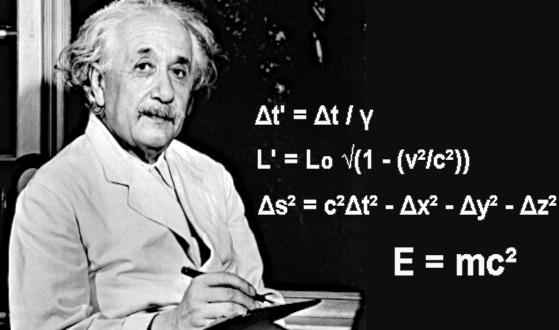


 Planck's wall theory
Planck's wall theory